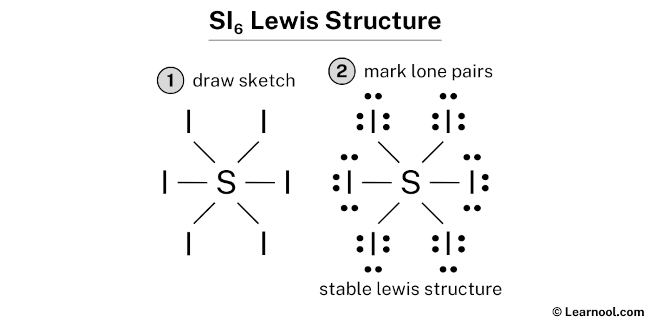
SI6 has one sulfur atom and six iodine atoms.
In SI6 Lewis structure, there are six single bonds around the sulfur atom, with six iodine atoms attached to it, and on each iodine atom, there are three lone pairs.
Steps
To properly draw the SI6 Lewis structure, follow these steps:
#1 Draw a rough sketch of the structure
#2 Next, indicate lone pairs on the atoms
#3 Indicate formal charges on the atoms, if necessary
Let’s break down each step in more detail.
#1 Draw a rough sketch of the structure
- First, determine the total number of valence electrons
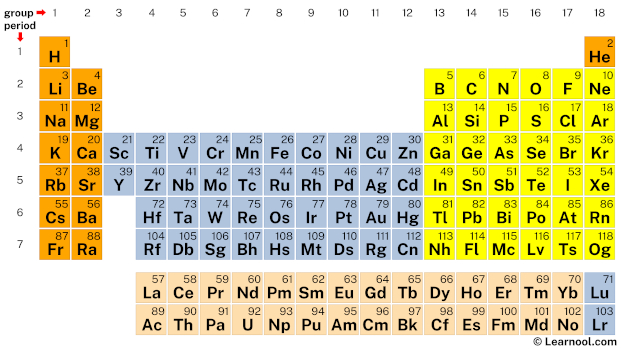
In the periodic table, sulfur lies in group 16, and iodine lies in group 17.
Hence, sulfur has six valence electrons and iodine has seven valence electrons.
Since SI6 has one sulfur atom and six iodine atoms, so…
Valence electrons of one sulfur atom = 6 × 1 = 6
Valence electrons of six iodine atoms = 7 × 6 = 42
And the total valence electrons = 6 + 42 = 48
Learn how to find: Sulfur valence electrons
- Second, find the total electron pairs
We have a total of 48 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Total electron pairs = total valence electrons ÷ 2
So the total electron pairs = 48 ÷ 2 = 24
- Third, determine the central atom
We have to place the least electronegative atom at the center.
Since sulfur is less electronegative than iodine, assume that the central atom is sulfur.
Therefore, place sulfur in the center and iodines on either side.
- And finally, draw the rough sketch
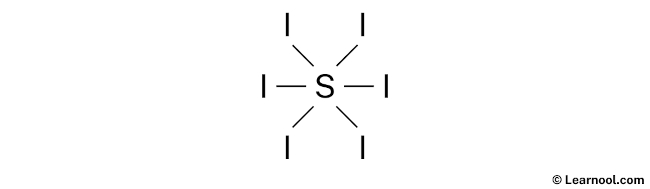
#2 Next, indicate lone pairs on the atoms
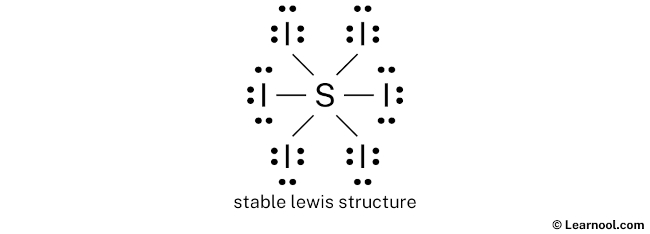
Here, we have a total of 24 electron pairs. And six S — I bonds are already marked. So we have to only mark the remaining eighteen electron pairs as lone pairs on the sketch.
Also remember that sulfur is a period 3 element, so it can keep more than 8 electrons in its last shell. And iodine is a period 5 element, so it can keep more than 8 electrons in its last shell.
Always start to mark the lone pairs from outside atoms. Here, the outside atoms are iodines.
So for each iodine, there are three lone pairs, and for sulfur, there is zero lone pair because all eighteen electron pairs are over.
Mark the lone pairs on the sketch as follows:
#3 Indicate formal charges on the atoms, if necessary
Use the following formula to calculate the formal charges on atoms:
Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons
For sulfur atom, formal charge = 6 – 0 – ½ (12) = 0
For each iodine atom, formal charge = 7 – 6 – ½ (2) = 0
Here, both sulfur and iodine atoms do not have charges, so no need to mark the charges.
In the above structure, you can see that the central atom (sulfur) forms an octet. And the outside atoms (iodines) also form an octet. Hence, the octet rule is satisfied.
Therefore, this structure is the stable Lewis structure of SI6.
Next: CBr2F2 Lewis structure
External links
- https://socratic.org/questions/what-is-the-molecular-geometry-of-si6-draw-its-vsepr-and-lewis-structure
- https://homework.study.com/explanation/what-is-the-molecular-geometry-of-si-6-draw-its-vsepr-and-lewis-structure.html
- https://oneclass.com/homework-help/chemistry/7037953-si6-lewis-structure.en.html
- https://www.numerade.com/ask/question/name-draw-the-best-lewis-dot-structure-for-each-of-the-following-species-give-the-molecular-geometry-and-shapes-of-these-molecules-using-vsepr-befz-6-bci-c-ccl-d-pbrs-e-si6-f-bh-g-ni-h-cif-1-41069/
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
