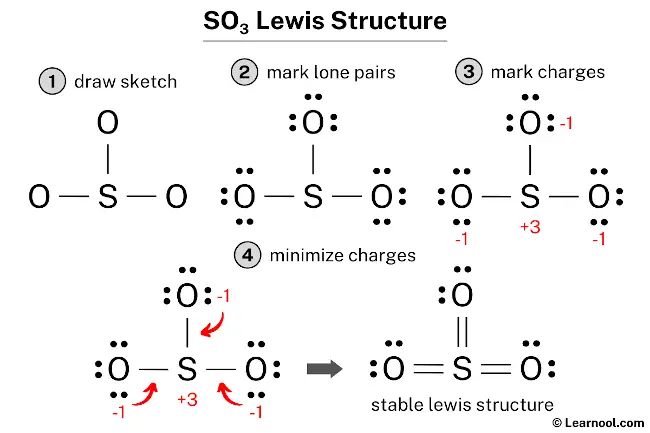
The SO3 Lewis structure illustrates how the atoms of sulfur trioxide, a compound composed of one sulfur atom and three oxygen atoms, are arranged. Within the SO3 Lewis structure, the sulfur atom is bonded to three oxygen atoms through double bonds. Each oxygen atom has two lone pairs of electrons associated with it.
To draw this structure, begin by sketching a rough diagram of the molecular arrangement. Next, indicate the lone pairs on each atom and check for any formal charges. If formal charges are present, convert lone pairs to bonds to minimize these charges. Repeat this process until all charges are minimized, ensuring that the octet rule is satisfied for both the central atom and the surrounding atoms. By following these steps, an accurate and stable SO3 Lewis structure can be drawn.
Alternative method: Lewis structure of SO3
Rough sketch
The initial step in sketching the SO3 Lewis structure is to determine the total number of valence electrons. Both sulfur and oxygen belong to group 16 of the periodic table, resulting in six valence electrons for each of them. The total number of valence electrons in the SO3 molecule can be calculated by adding up the valence electrons of each atom. The sulfur atom and each of the three oxygen atoms have six valence electrons, resulting in a total of 6 + 18 = 24 valence electrons in the SO3 molecule.
Learn how to find: Sulfur valence electrons and Oxygen valence electrons
The second step in sketching the SO3 Lewis structure is to determine the total number of electron pairs. To find the total electron pairs in the SO3 molecule, divide the total number of valence electrons by two. In this case, there are 24 valence electrons, so the total electron pairs can be calculated as 24 ÷ 2 = 12.
To proceed with the SO3 Lewis structure, the next step is to determine the central atom. The least electronegative atom is typically placed at the center. In the case of SO3, sulfur has a lower electronegativity than oxygen. Therefore, sulfur is placed at the center, and the three oxygen atoms are arranged around it. Once the central atom has been determined, the next step is to draw a rough sketch of the molecule.
Lone pair
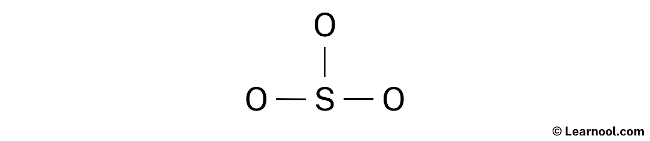
To locate the lone pairs on the atoms, it is necessary to refer to the rough sketch of the SO3 Lewis structure previously drawn. With a total of 12 electron pairs in the molecule and three sulfur-oxygen double bonds already shown in the sketch, which utilize six electron pairs, the remaining nine electron pairs should be marked as lone pairs on the sketch.
Keep in mind that sulfur belongs to period 3 of the periodic table, which means it can accommodate more than 8 electrons in its outermost shell. On the other hand, oxygen belongs to period 2 of the periodic table and can not hold more than 8 electrons in its outermost shell.
When marking the lone pairs, it is advisable to begin with the outside atoms first. In this case, the outside atoms are the three oxygen atoms. Each oxygen atom will receive three lone pairs, whereas the central sulfur atom will not receive lone pairs because all nine electron pairs are accounted for. Therefore, the lone pairs should be marked on the sketch in the respective manner.
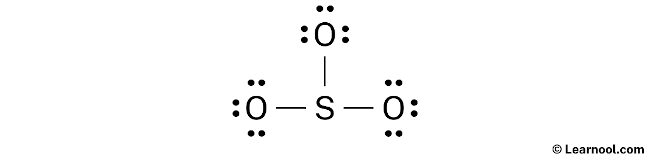
Formal charge
To assign formal charges to the atoms in the SO3 molecule, use the formula: Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons. For the sulfur atom, the formal charge can be calculated as 6 – 0 – ½ (6) = +3, while for each oxygen atom, the formal charge can be calculated as 6 – 6 – ½ (2) = -1.
It is important to note that both the sulfur and oxygen atoms carry charges, and thus, it is necessary to indicate them on the sketch.
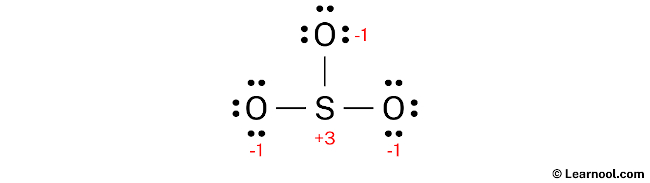
The current Lewis structure is not stable as both the sulfur and oxygen atoms have charges. In order to attain stability, the charges must be minimized by converting some of the lone pairs into bonds.
One way to minimize the formal charges in the structure is by converting lone pairs of the oxygen atoms into new S – O bonds. This will result in a more stable Lewis structure by reducing the formal charges on both the sulfur and oxygen atoms.
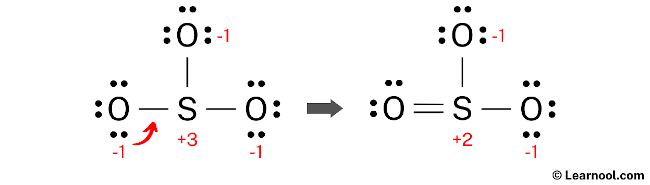
To further reduce the formal charges on the sulfur and oxygen atoms, it is necessary to convert another lone pair of one of the oxygen atoms into a new S – O bond with the sulfur atom. This will result in a more stable Lewis structure, as the formal charges will be minimized.
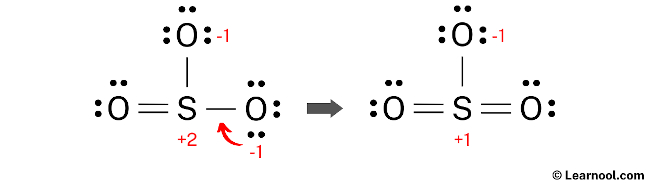
Formal charges continue to exist in the current SO3 Lewis structure. To further minimize the formal charges on both sulfur and oxygen atoms, it is advisable to convert an additional lone pair of an oxygen atom into a new S – O bond with the sulfur atom.
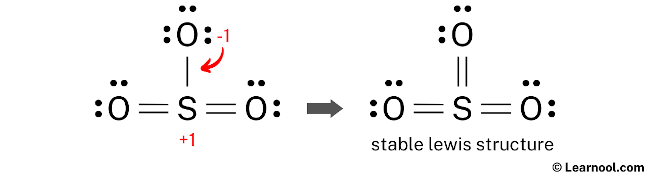
Final structure
The final structure of SO3 features a central sulfur atom connected to three oxygen atoms. In this configuration, the sulfur atom serves as an exception to the octet rule, utilizing an expanded valence shell to accommodate twelve electrons through three double bonds, one with each oxygen atom. Each oxygen atom fulfills its octet by retaining two lone pairs alongside its double bond with the sulfur. This arrangement is the most stable because it results in formal charges of zero for every atom in the molecule, representing the most energetically favorable state. Accordingly, this specific electronic distribution serves as the definitive and most accurate Lewis representation of sulfur trioxide.
Next: C2H4 Lewis structure
External video
- Lewis Dot Structure for SO3 (Sulfur trioxide) – YouTube • Wayne Breslyn
External links
- The Lewis structure of Sulfur trioxide – ChemicalBook
- Lewis Structure for SO3 (Sulfur Trioxide) – The University of Maryland
- How to Draw the Lewis Structure for SO3 (Sulfur Trioxide) – Pinterest
- How to determine the Lewis dot structure of SO3 – Quora
- Lewis structure of SO3:Biochemhelp – AceOrganicChem
- What is the Lewis structure of SO3? – Homework.Study.com
- draw the lewis structure for the sulfur trioxide so3 molecule – Brainly
- SO3 Lewis Structure – Laurence Lavelle
- What are all resonance structures for SO3? – Socratic
- Draw a single Lewis structure for an SO3 molecule – Chegg
- Sulfur Trioxide (SO3) Formal Charge – Chemistry Learner
- Chemical Bonding: SO3 Lewis Structure – The Geoexchange
- Lewis Dot of Sulfur Trioxide SO3 – Kent’s Chemistry
- Sulfur Trioxide (SO3) Lewis Structure, Hybridization – Chemistry School
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
