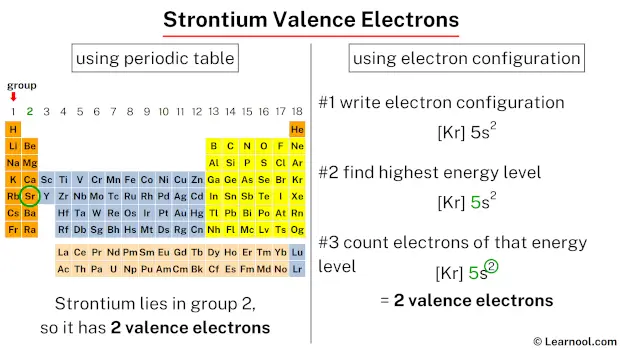
Strontium, classified as an alkaline earth metal, possesses 2 valence electrons. To determine the valence electrons for strontium, one can refer to its position on the periodic table, like which group it is located in, or simply check its electron configuration.
Methods
Using periodic table
Get the periodic table having the chemical elements marked on it as mentioned above.
Now mark the location of strontium on the periodic table.
Next, mark the group number of strontium on the periodic table.
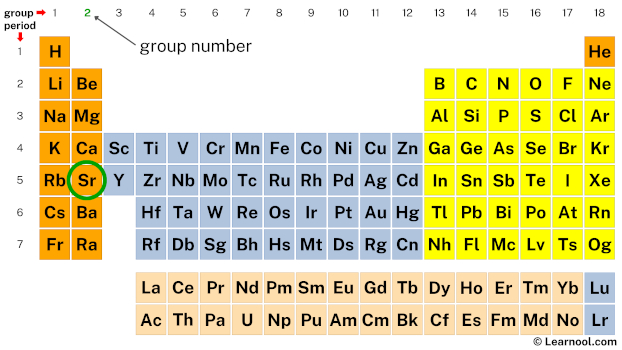
The valence electrons of each main-group element can be determined by the column in which it is located.
(i.e., all group 1 elements have 1 valence electron, all group 2 elements have 2 valence electrons, skip the transition metals… then, all group 13 elements have 3 valence electrons, all group 14 elements have 4 valence electrons, and so on up to group 18 elements)
Since strontium is in group 2, it has 2 valence electrons.
Using electron configuration
- First, write electron configuration of strontium
The electron configuration of strontium is [Kr] 5s2.
Learn how to find: Strontium electron configuration
- Second, find highest energy level in electron configuration
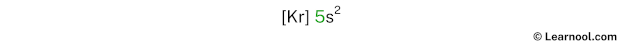
In the above electron configuration, the highest energy level (5) is marked with green color.
- Finally, count electrons of that energy level
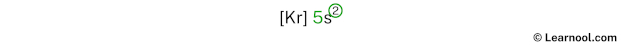
The 5th energy level contains 5s subshell and it has 2 electrons. So strontium has a total of 2 valence electrons.
Next: Cesium valence electrons
Related
More topics
- Strontium
- Strontium Bohr model
- Strontium electron configuration
- Strontium orbital diagram
- Strontium protons neutrons electrons
External links
- https://homework.study.com/explanation/how-many-valence-electrons-does-strontium-have.html
- https://valenceelectrons.com/valence-electrons-of-strontium/
- https://materials.gelsonluz.com/2019/08/valence-electrons-in-strontium-sr-facts.html
- https://brainly.com/question/11324321
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
