Thermal energy, also referred to as heat energy, is a form of energy that arises from the movement and collisions of atoms and molecules within a heated substance. The increase in thermal energy is a result of the substance’s elevated temperature, which causes its constituent particles to gain kinetic energy and exhibit more rapid motion. As the temperature rises, so does the thermal energy of the substance.
Examples
Hot tea

Hot tea contains thermal energy due to its elevated temperature. Thermal energy is the kinetic energy of particles within a substance, and in the case of hot tea, the water molecules are moving rapidly, indicating a higher temperature. This energy can be transferred to other objects or converted into other forms, such as steam when the tea boils. The warmth you feel while holding a cup of hot tea is a direct experience of its thermal energy being transferred to your skin.
Bathtub

The bathtub filled with hot water possesses thermal energy because of the increased temperature of the water. Thermal energy, a form of kinetic energy at the molecular level, is evident in the fast movement of water molecules when heated. The warmth you sense when immersed in the hot water is a manifestation of this thermal energy being transferred to your body. Additionally, the thermal energy in the hot water can be transferred to the surrounding environment, influencing the temperature of the room.
Hot pizza slice

The piping hot pizza slice holds thermal energy gained during the cooking process. Thermal energy is a result of the high temperature of the pizza, where heat is transferred to the food, causing the molecules in the pizza to move rapidly. This kinetic energy is what we perceive as warmth when we touch or consume the pizza. The thermal energy in the hot pizza slice gradually dissipates into the surrounding environment as it cools, highlighting the dynamic nature of thermal energy transfer.
Sun rays

Sunlight radiates warmth through the emission of radiant heat, representing a transfer of thermal energy to the surrounding environment. This form of energy, transmitted as electromagnetic waves, reaches Earth and imparts thermal energy to the atmosphere, surfaces, and natural phenomena like winds and ocean currents. The sensation of warmth on a sunny day directly arises from the Sun’s radiant heat, showcasing the interconnected dynamics of energy transfer in influencing Earth’s climate and temperature.
Boiling water

The vessel of boiling water on a stove demonstrates thermal energy as the water molecules within it acquire kinetic energy and heat up. When heat is applied to the stove, the energy is transferred to the water, causing its molecules to move rapidly. This increased molecular motion signifies a rise in temperature, and when the water reaches its boiling point, it undergoes a phase change into steam. The bubbling and visible steam are visible manifestations of the thermal energy present, illustrating the dynamic relationship between heat input, molecular motion, and the observable effects of boiling water.
Cooked food

Cooked food taken from a microwave oven maintains thermal energy acquired during the heating process. In the microwave, electromagnetic waves excite water molecules and other components in the food, leading to increased kinetic energy. This heightened molecular movement results in a higher temperature, thus cooking the food. When you retrieve the cooked food, it still holds thermal energy, and the warmth you feel is a direct result of this retained energy. The process showcases how microwave ovens efficiently transfer thermal energy to cook food quickly and conveniently.
Drinking water

The ice cube undergoes a transformation when exposed to water, demonstrating the transfer of thermal energy. As the warmer water interacts with the ice, it imparts heat, causing the ice molecules to gain kinetic energy and shift from a solid to a liquid state. This alteration in the ice’s physical state highlights the dynamic exchange of thermal energy between the water and the ice.
Hands

When you hold a cold glass of juice, your hand transfers thermal energy to the glass, raising its temperature and making it more comfortable to hold. The heat from your hand is transferred to the cooler glass, and the glass, in turn, absorbs this thermal energy, causing a warming effect. This process is an example of heat transfer through conduction, where the warmer object (your hand) imparts thermal energy to the cooler object (the cold glass), ultimately reaching a balance in temperature.
Matchstick

The ignited matchstick produces heat through a chemical reaction, generating thermal energy. The process involves the combustion of the match head, where chemicals react with oxygen, releasing heat energy in the form of a flame. This thermal energy is a manifestation of the exothermic reaction occurring during the combustion process. The heat generated not only sustains the flame but can also be transferred to nearby objects, illustrating the conversion of chemical energy into thermal energy during the ignition of a matchstick.
Heater

In a heater, the conversion of electrical energy into heat results in the generation of thermal energy, which is then radiated into the room, providing warmth to the surrounding space. The effective transfer of this thermal energy from the heater to the air and nearby surfaces is what contributes to the comforting temperature you feel. This operational mechanism highlights how heaters are structured to efficiently distribute thermal energy, ensuring a cozy environment in colder conditions.
Skillet

As the skillet sits on a stove, it absorbs heat from the burner, gaining thermal energy essential for cooking food. This heat absorption is crucial for the cooking process, as it raises the temperature of the skillet, facilitating the transfer of thermal energy to the food placed within it. The interaction between the skillet and the stove burner exemplifies how thermal energy is harnessed in cooking, influencing the texture and flavor of the food being prepared.
Toast

The piece of toast emerging from a toaster carries thermal energy. It has been heated to a desired level of crispiness during the toasting process. The heat applied to the bread causes its molecules to undergo Maillard reactions, resulting in browning and the distinctive toasted flavor. This transformation illustrates the conversion of electrical energy in the toaster into thermal energy, altering the texture and taste of the bread. The warmth you feel when handling the toast is a direct consequence of the thermal energy imparted to it during toasting.
Equation
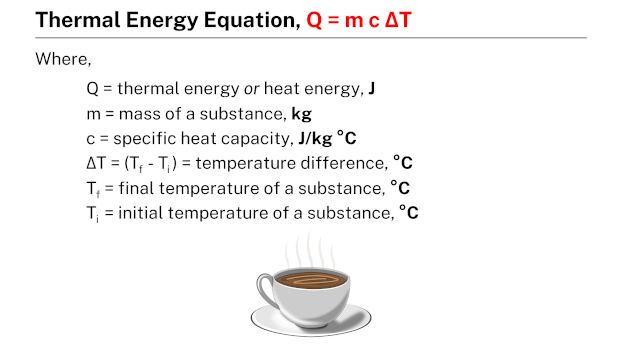
The thermal energy equation, Q = m c ΔT, establishes a relationship between the thermal energy (Q) of a substance and its mass (m), specific heat (c), and the temperature difference (ΔT). This equation enables the calculation of thermal energy by considering the mass of the substance, its specific heat, and the change in temperature.
Practice problems
Problem #1
Calculate the thermal energy required to raise the temperature of 1.5 kg of oil from 10 ℃ to 90 ℃, given that the specific heat of oil is 2.1 J/kg ℃.
Solution
Given data:
- Thermal energy, Q = ?
- Mass of oil, m = 1.5 kg
- Initial temperature of oil, Ti = 10 ℃
- Final temperature of oil, Tf = 90 ℃
- Therefore, the temperature difference, ΔT = Tf – Ti = 80 ℃
- Specific heat of oil, c = 2.1 J/kg ℃
Applying the formula:
- Q = m c ΔT
- Q = 1.5 × 2.1 × 80
- Q = 252 J
Therefore, the thermal energy required to raise the temperature of oil is 252 J.
Problem #2
When 500 g of a substance is heated, its temperature increases from 20 ℃ to 70 ℃. Calculate the thermal energy required to raise the temperature of the substance, considering its specific heat of 4.8 J/kg ℃.
Solution
Given data:
- Mass of a substance, m = 500 g = 0.5 kg
- Initial temperature, Ti = 20 ℃
- Final temperature, Tf = 70 ℃
- Therefore, the temperature difference, ΔT = Tf – Ti = 50 ℃
- Specific heat, c = 4.8 J/kg ℃
- Thermal energy, Q = ?
Applying the formula:
- Q = m c ΔT
- Q = 0.5 × 4.8 × 50
- Q = 120 J
Therefore, the thermal energy required to raise the temperature of a substance is 120 J.
Problem #3
A 750 g sample of a material is heated, causing its temperature to increase from 35 ℃ to 85 ℃. Calculate the amount of thermal energy required to raise the temperature of the material, given that its specific heat is 1.6 J/kg ℃.
Solution
Given data:
- Mass of a material, m = 750 g = 0.75 kg
- Initial temperature, Ti = 35 ℃
- Final temperature, Tf = 85 ℃
- Therefore, the temperature difference, ΔT = Tf – Ti = 50 ℃
- Thermal energy, Q = ?
- Specific heat, c = 1.6 J/kg ℃
Applying the formula:
- Q = m c ΔT
- Q = 0.75 × 1.6 × 50
- Q = 60 J
Therefore, the thermal energy required to raise the temperature of a material is 60 J.
Problem #4
Calculate the thermal energy needed to raise the temperature of a 2 kg wooden object from 24 ℃ to 64 ℃. The specific heat of wood is 3.4 J/kg ℃.
Solution
Given data:
- Thermal energy, Q = ?
- Mass of a wooden object, m = 2 kg
- Initial temperature, Ti = 24 ℃
- Final temperature, Tf = 64 ℃
- Therefore, the temperature difference, ΔT = Tf – Ti = 40 ℃
- Specific heat, c = 3.4 J/kg ℃
Applying the formula:
- Q = m c ΔT
- Q = 2 × 3.4 × 40
- Q = 272 J
Therefore, the thermal energy required to raise the temperature of a wooden object is 272 J.
Related
More topics
- Thermal energy
- Potential energy
- Kinetic energy
- Chemical energy
- Electrical energy
- Geothermal energy
- Radiant energy
- Sound energy
- Elastic energy
- Gravitational energy
- Mechanical energy
- Electric potential energy
- Rotational energy
- Photon energy
External links
- https://www.solarschools.net/knowledge-bank/energy/types/thermal
- https://study.com/academy/lesson/what-is-thermal-energy-definition-examples.html
- https://www.khanacademy.org/science/physics/work-and-energy/work-and-energy-tutorial/a/what-is-thermal-energy
- https://justenergy.com/blog/thermal-energy-what-it-is-how-it-works-environmental-impact/
- https://examples.yourdictionary.com/examples-of-heat-energy.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
