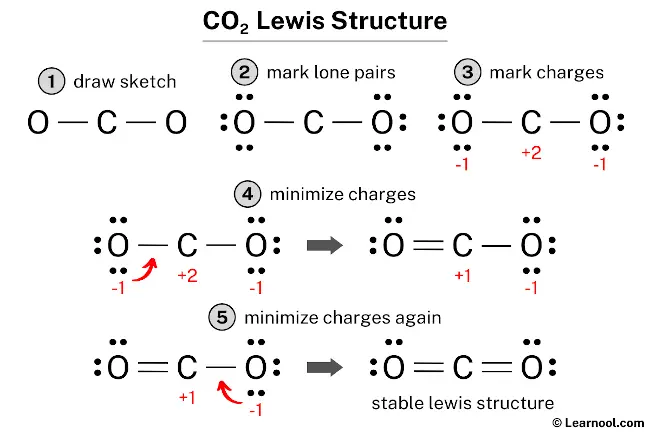
The CO2 Lewis structure depicts the molecular arrangement of carbon dioxide, which is composed of one carbon atom and two oxygen atoms. Within the CO2 Lewis structure, the carbon atom is surrounded by two double bonds, with each oxygen atom attached to it. Each oxygen atom possesses two lone pairs, while the carbon atom does not have any lone pairs.
To draw a CO2 Lewis structure, begin by sketching out a rough structure of the molecule and indicating the locations of any lone pairs on the atoms. If necessary, formal charges on the atoms should be indicated next. Then, minimize these formal charges by converting the lone pairs of atoms to chemical bonds. Repeat this step until all charges are minimized, ensuring that the octet rule is satisfied for both the central atom and the outer atoms. By following these steps, an accurate and complete CO2 Lewis structure can be drawn.
Steps
Sketch the structure
To sketch the CO2 Lewis structure, the first step is to determine the total number of valence electrons. The total valence electrons in the molecule can be calculated by multiplying the valence electrons of each atom. Carbon, which belongs to group 14 of the periodic table, has four valence electrons, while oxygen, belonging to group 16, has six valence electrons. Since CO2 consists of one carbon atom and two oxygen atoms, the valence electrons for one carbon atom is 4, and for two oxygen atoms, it is 12, resulting in a total of 4 + 12, which equals 16 valence electrons.
Learn how to find: Carbon valence electrons and Oxygen valence electrons
After determining the total number of valence electrons, the next step in sketching the CO2 Lewis structure is to find the total electron pairs. This can be done by dividing the total number of valence electrons by two. In this case, with 16 valence electrons, the total electron pairs would be 16 divided by 2, which equals 8.
Once the electron pairs have been identified, the next step is to determine the central atom. It is recommended to place the least electronegative atom at the center, and in the case of CO2, carbon is less electronegative than oxygen. Therefore, it is assumed that the central atom is carbon. Once the central atom has been determined, it can be placed in the center with the two oxygen atoms on either side. With the central atom in place, the rough sketch of the CO2 Lewis structure can now be drawn.
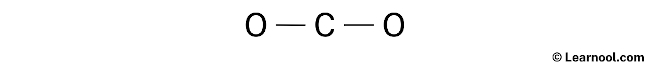
Indicate lone pair
After sketching the rough structure, the next step in drawing the CO2 Lewis structure is to indicate the lone pairs. In total, there are eight electron pairs, with two C – O bonds already marked. These two C – O bonds utilize four electron pairs. Therefore, the remaining six electron pairs should be marked as lone pairs on the sketch.
It is important to remember that both carbon and oxygen are period 2 elements, meaning they cannot have more than 8 electrons in their outer shell.
To begin marking the lone pairs, start with the outside atoms, in this case, the oxygen atoms. Each oxygen will have three lone pairs, while the carbon will have no lone pairs because all six electron pairs are already accounted for. Mark the lone pairs on the sketch accordingly.
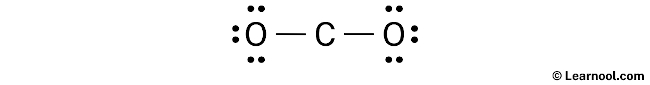
Assign formal charge
To calculate the formal charges on the atoms in CO2, use the formula: Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons. For the carbon atom, the formal charge is calculated as 4 – 0 – ½ (4) = +2, while for each oxygen atom, the formal charge is 6 – 6 – ½ (2) = -1.
After calculating the formal charges, it is important to mark them on the sketch since both the carbon and oxygen atoms have charges.
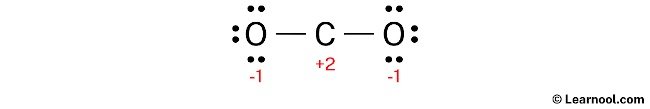
The Lewis structure is not considered stable because of the presence of charges on both the carbon and oxygen atoms. To minimize these charges, it is necessary to convert lone pairs into bonds.
Minimize formal charge
To minimize formal charges, a lone pair of one of the oxygen atoms can be converted into a new C – O bond with the carbon atom.

To further minimize formal charges, it is necessary to convert another lone pair of the oxygen atom to form a new C – O bond with the carbon atom. This will help to reduce the formal charges on the atoms and make the Lewis structure more stable.
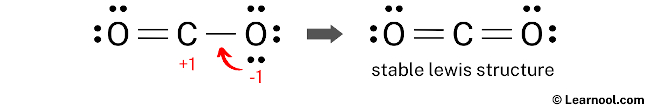
The Lewis structure of CO2 satisfies the octet rule, with both the central carbon atom and the outside oxygen atoms having a full octet of electrons. This arrangement results in a more stable structure compared to previous ones. Therefore, this particular structure represents the stable Lewis structure of CO2.
Next: SO2 Lewis structure
External video
External links
- CO2 Lewis Structure – Learn With Dr. Scott
- The Lewis structure of Carbon dioxide – ChemicalBook
- Lewis Structure of CO2 [with video and free study guide] – AceOrganicChem
- How can I draw the Lewis structure for CO2? – Socratic
- Is this Lewis structure for CO2 correct? I thought CO2 is only made of double bonds?? – Reddit
- File:Carbon-dioxide-octet-dot-cross-2D.png – Wikimedia Commons
- How many pairs of electrons should be drawn in the Lewis structure for carbon dioxide (Co2)? – Quora
- Chemical Bonding: CO2 Lewis Structure – The Geoexchange
- Draw the Lewis structure for CO2. How many single, double, and triple covalent bonds are present in this molecule? What is its molecular geometry? Is it polar or nonpolar? – Homework.Study.com
- co2 lewis structure – LinkedIn
- CO2 Lewis Structure (Carbon Dioxide) – Pinterest
- What is the correct Lewis structure for CO2? – Chegg
- Carbon dioxide – Wikipedia
- Which of the following is the Lewis structure for CO2? – Brainly
- What is the Lewis structure for carbon dioxide? – CK-12 Foundation
- Lewis Dot of Carbon Dioxide CO2 – Kent’s Chemistry
- Molecular Geometry of Carbon Dioxide (CO2) – Chemistry Learner
- Lewis dot structure CO2 – ShowMe
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
