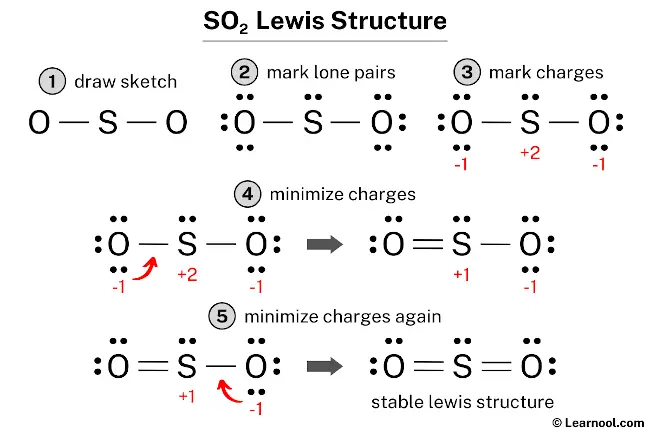
The SO2 Lewis structure depicts the molecular arrangement of sulfur dioxide, which consists of one sulfur atom and two oxygen atoms. In the SO2 Lewis structure, there is a double bond between the sulfur atom and each oxygen atom. Each oxygen atom possesses two lone pairs, while the sulfur atom has one lone pair.
To draw the SO2 Lewis structure correctly, begin by sketching the molecule and marking the lone pairs on the atoms. Next, calculate and mark the formal charges on the atoms and make necessary adjustments by converting lone pairs of the atoms to chemical bonds. Repeat the process until all formal charges are minimized and try to achieve a stable Lewis structure with the least possible formal charges. These steps ensure the correct distribution of electrons and the satisfaction of the octet rule for all atoms in the SO2 molecule.
Steps
Sketch the structure
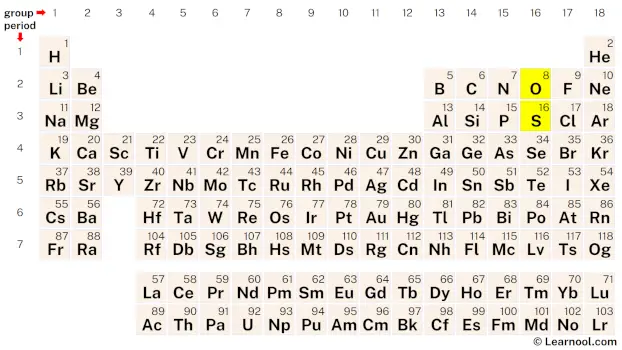
The first step in sketching the SO2 structure is to determine the total number of valence electrons. Since both sulfur and oxygen are in group 16 of the periodic table, they each have six valence electrons. As SO2 has one sulfur atom and two oxygen atoms, the total number of valence electrons can be calculated by adding up the valence electrons of each atom: 6 valence electrons for the sulfur atom and 12 valence electrons for the two oxygen atoms. Therefore, the total number of valence electrons in the SO2 molecule is 6 + 12 = 18.
Learn how to find: Sulfur valence electrons and Oxygen valence electrons
The second step in sketching the SO2 structure is to determine the total number of electron pairs. To calculate this, divide the total number of valence electrons by 2. In the case of SO2, there are 18 valence electrons. Thus, the total electron pairs can be found by dividing 18 by 2, which gives a result of 9 electron pairs.
Once the total electron pairs have been identified, the next step is to determine the central atom for the SO2 molecule. The least electronegative atom is usually placed at the center, and in this case, sulfur has a lower electronegativity than oxygen. Therefore, assume that the central atom is sulfur and place it in the center, with one oxygen atom on either side. After determining the positions of the atoms, a rough sketch of the SO2 structure can be drawn.
Indicate lone pair
Once the rough sketch of the SO2 structure has been drawn, the next step is to identify and indicate the lone pairs on the atoms. It is important to note that there are a total of 9 electron pairs for the molecule, with two S – O bonds already shown in the sketch, utilizing four electron pairs. Therefore, the remaining seven electron pairs should be marked as lone pairs.
It’s worth noting that sulfur is a period 3 element, meaning it can accommodate more than 8 electrons in its valence shell. On the other hand, oxygen is a period 2 element, meaning it cannot hold more than 8 electrons in its valence shell.
To correctly indicate the lone pairs in the SO2 structure, it is important to start from the outside atoms, which in this case are the two oxygen atoms. Each oxygen atom will receive three lone pairs, while the central sulfur atom will have only one lone pair. These lone pairs can be marked on the rough sketch of the structure according to their respective atoms.
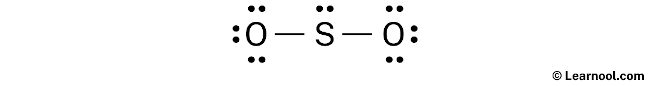
Assign formal charge
To assign formal charges to the atoms in the SO2 molecule, use the formula: Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons. The valence electrons for sulfur is 6, while it has 2 nonbonding electrons and 4 bonding electrons. Plugging these values into the formula gives a formal charge of +2 for the sulfur atom. For each oxygen atom, the valence electrons are 6, and there are 6 nonbonding electrons and 2 bonding electrons, leading to a formal charge of -1.
As both the sulfur and oxygen atoms have charges, they should be marked on the sketch to clearly indicate their formal charges.
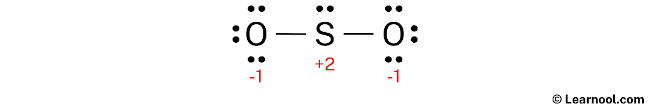
The current Lewis structure is not stable as it has charges on both sulfur and oxygen atoms. In order to obtain a stable structure, it is necessary to reduce the formal charges by converting lone pairs to bonds.
Minimize formal charge
To reduce the formal charges on the SO2 structure, one approach is to convert a lone pair of one of the oxygen atoms into a new bond with the sulfur atom. This will help to reduce the formal charges on the atoms and make the structure more stable.

To further minimize the formal charges on the SO2 structure, it is necessary to convert another lone pair of the oxygen atom into a new bond with the sulfur atom. This will help to reduce the charges on the atoms and increase the stability of the structure.
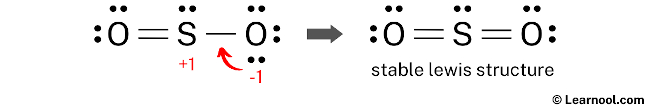
The Lewis structure for SO2 satisfies the octet rule as the central sulfur atom and the two surrounding oxygen atoms each have eight electrons in their valence shell. This arrangement results in a more stable structure compared to previous attempts. Therefore, this final structure represents the stable Lewis structure of SO2.
Next: NO2 Lewis structure
External video
External links
- How the SO2 lewis structure is formed – ChemicalBook
- Draw the Lewis structure for SO2 – Homework.Study.com
- What’s the lewis structure for “SO2”? – Reddit
- What is the lewis structure for SO2? – Socratic
- draw the lewis structure for sulfur dioxide, so2 – Brainly
- Lewis structure of SO2 – Laurence Lavelle
- SO2 Lewis Structure – Pinterest
- Lewis Structure of SO2 – Chemistry Stack Exchange
- Why does Sulphur in SO2 have 10 electron in the Lewis Structure? – Quora
- Lewis Dot of Sulfur Dioxide SO2 – Kent’s Chemistry
- How many electrons are in the Lewis structure for SO2? – Brainly
- Chemical Bonding: SO2 Lewis Structure – The Geoexchange
- draw a lewis structure for so2 in which all atoms obey the octet rule. show formal charges – Quizlet
- Sulfur Dioxide (SO2) Lewis Structure – Physics Forums
- Draw the Lewis Structure for sulfur dioxide – Chegg
- Sulfur dioxide Lewis structure and formal charge – ShowMe
- Molecular Geometry, Lewis Structure, and Bond Angle SO2 – Chemistry Learner
- Lewis dot so2? – Answers
- Sulfur Dioxide | SO2 | CID 1119 – National Institutes of Health (.gov)
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
