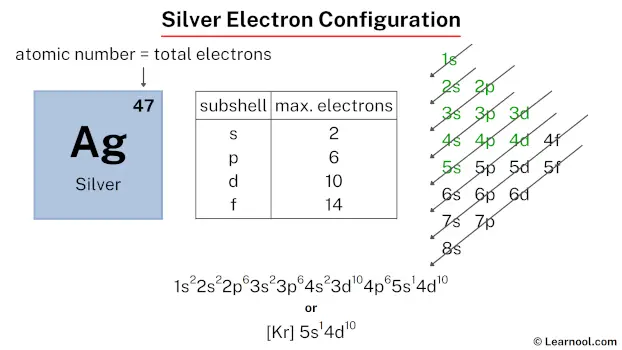
The silver electron configuration, represented as [Kr] 5s1 4d10 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10, illustrates the precise arrangement of electrons within the atom. This configuration can be determined through various methods, including the aufbau principle, periodic table organization, Bohr model representation, or orbital diagram visualization.
Methods
Aufbau principle
- First, find electrons of silver atom
The atomic number of silver represents the total number of electrons of silver. Since the atomic number of silver is 47, the total electrons of silver are 47.
- Second, make a table of subshell and its maximum electrons
Calculate the maximum number of electrons each subshell can hold using the formula: 4ℓ + 2
Where, ℓ = azimuthal quantum number of the subshell
For s subshell, ℓ = 0
For p subshell, ℓ = 1
For d subshell, ℓ = 2
For f subshell, ℓ = 3
| subshell | max. electrons |
| s | 2 |
| p | 6 |
| d | 10 |
| f | 14 |
This means that,
Each s subshell can hold maximum 2 electrons
Each p subshell can hold maximum 6 electrons
Each d subshell can hold maximum 10 electrons
Each f subshell can hold maximum 14 electrons
- Finally, use aufbau chart and start writing electron configuration
Remember that we have a total of 47 electrons.
According to the aufbau principle, 1s subshell is filled first and then 2s, 2p, 3s… and so on.
By looking at the chart, you can see that electrons are first filled in 1s subshell. Each s-subshell can hold a maximum of 2 electrons, so we will use 2 electrons for the 1s subshell.
So the electron configuration will be 1s2. Where, 1s2 indicates that the 1s subshell has 2 electrons.
Now we have used 2 electrons in the 1s subshell, so we have a total of 47 – 2 = 45 electrons left.
Looking at the chart, after 1s subshell now comes 2s subshell. Again, each s-subshell can hold a maximum of 2 electrons, so we will use 2 electrons for the 2s subshell.
So the electron configuration will be 1s2 2s2. Where, 2s2 indicates that the 2s subshell has 2 electrons.
Again, we have used 2 electrons in the 2s subshell, so we have a total of 45 – 2 = 43 electrons left.
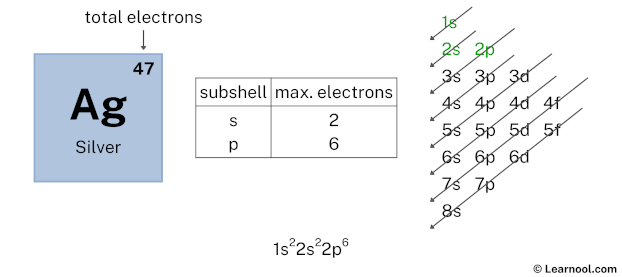
After 2s subshell now comes 2p subshell. Each p-subshell can hold a maximum of 6 electrons, so we will use 6 electrons for the 2p subshell.
So the electron configuration will be 1s2 2s2 2p6. Where, 2p6 indicates that the 2p subshell has 6 electrons.
Here, we have used 6 electrons in the 2p subshell, so we have a total of 43 – 6 = 37 electrons left.
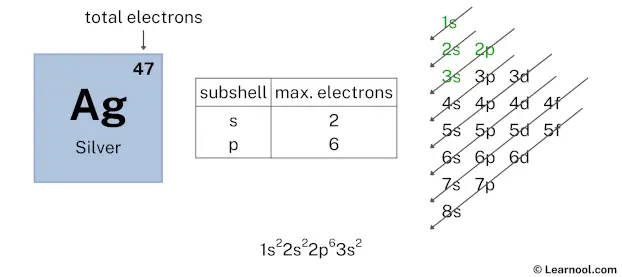
After 2p subshell now comes 3s subshell. Each s-subshell can hold a maximum of 2 electrons, so we will use 2 electrons for the 3s subshell.
So the electron configuration will be 1s2 2s2 2p6 3s2. Where, 3s2 indicates that the 3s subshell has 2 electrons.
Here, we have used 2 electrons in the 3s subshell, so we have a total of 37 – 2 = 35 electrons left.
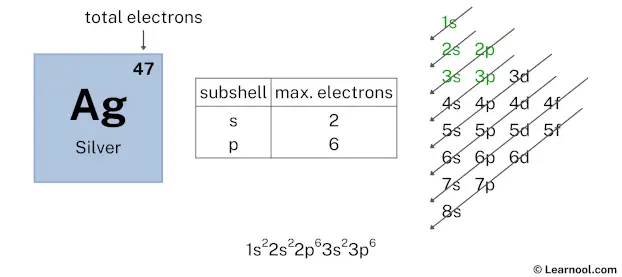
After 3s subshell now comes 3p subshell. Each p-subshell can hold a maximum of 6 electrons, so we will use 6 electrons for the 3p subshell.
So the electron configuration will be 1s2 2s2 2p6 3s2 3p6. Where, 3p6 indicates that the 3p subshell has 6 electrons.
Here, we have used 6 electrons in the 3p subshell, so we have a total of 35 – 6 = 29 electrons left.
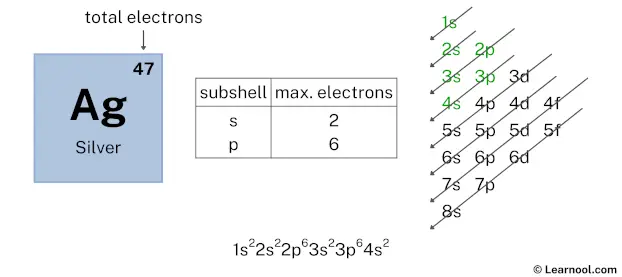
After 3p subshell now comes 4s subshell. Each s-subshell can hold a maximum of 2 electrons, so we will use 2 electrons for the 4s subshell.
So the electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2. Where, 4s2 indicates that the 4s subshell has 2 electrons.
Here, we have used 2 electrons in the 4s subshell, so we have a total of 29 – 2 = 27 electrons left.

After 4s subshell now comes 3d subshell. Each d-subshell can hold a maximum of 10 electrons, so we will use 10 electrons for the 3d subshell.
So the electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Where, 3d10 indicates that the 3d subshell has 10 electrons.
Here, we have used 10 electrons in the 3d subshell, so we have a total of 27 – 10 = 17 electrons left.
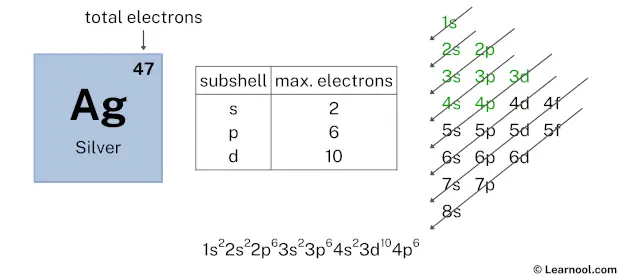
After 3d subshell now comes 4p subshell. Each p-subshell can hold a maximum of 6 electrons, so we will use 6 electrons for the 4p subshell.
So the electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. Where, 4p6 indicates that the 4p subshell has 6 electrons.
Here, we have used 6 electrons in the 4p subshell, so we have a total of 17 – 6 = 11 electrons left.
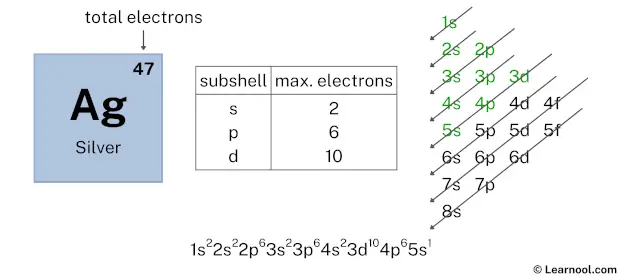
After 4p subshell now comes 5s subshell. Each s-subshell can hold a maximum of 2 electrons, but here we will use only 1 electron for the 5s subshell. Reason explained below.
So the electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1. Where, 5s1 indicates that the 5s subshell has 1 electron.
Here, we have used 1 electron in the 5s subshell, so we have a total of 11 – 1 = 10 electrons left.
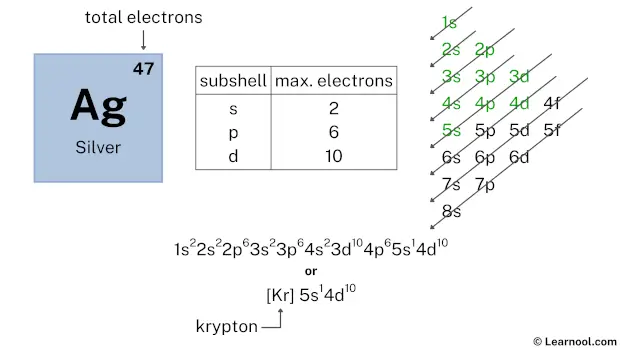
After 5s subshell now comes 4d subshell. Each d-subshell can hold a maximum of 10 electrons, and we also have 10 electrons left, so we will use that 10 electrons for the 4d subshell.
So the electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10. Where, 4d10 indicates that the 4d subshell has 10 electrons.
Therefore, the final electron configuration of silver is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10. And the condensed/abbreviated electron configuration of silver is [Kr] 5s1 4d10.
Where, Kr is krypton
Question: Why does the 5s subshell have only one electron (instead of two electrons), and the 4d subshell has ten electrons (instead of nine electrons)?
Let’s draw the orbital diagram of silver, and find the answer to the above question.
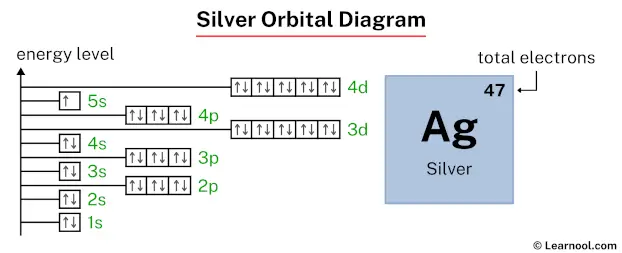
Answer: A completely full 4d subshell is more stable than a partially filled 4d subshell. That’s why the 4d subshell has ten electrons (instead of nine electrons). And the 5s subshell has one electron (instead of two electrons).
Periodic table
- First, get periodic table chart with spdf notation
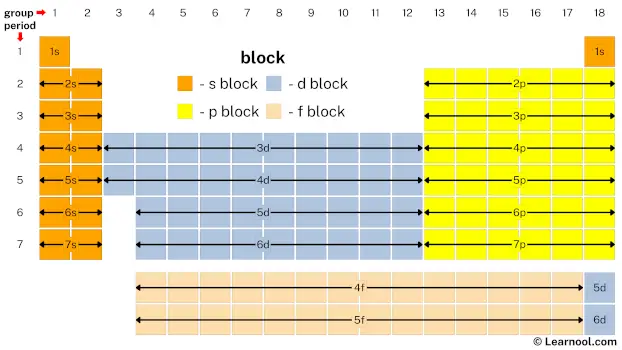
The above image shows periodic table blocks.
The ‘s’ in s block represents that all s block elements have their valence electrons in s subshell. Similarly, the ‘p’ in p block represents that all p block elements have their valence electrons in p subshell. And so on for d block and f block.
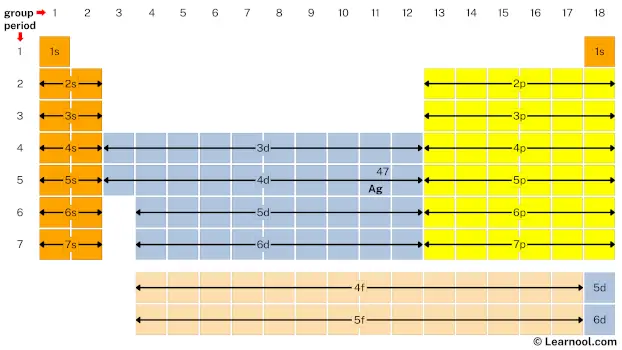
- Second, mark location of silver on periodic table
Silver is the d block element located in group 11 and period 5. Hence, mark the location of silver on the periodic table as follows:
- Finally, start writing electron configuration
Remember that: each s subshell can hold maximum 2 electrons, each p subshell can hold maximum 6 electrons, each d subshell can hold maximum 10 electrons, and each f subshell can hold maximum 14 electrons.
Start writing electron configuration from the very first element (i.e., hydrogen) all the way up to silver.
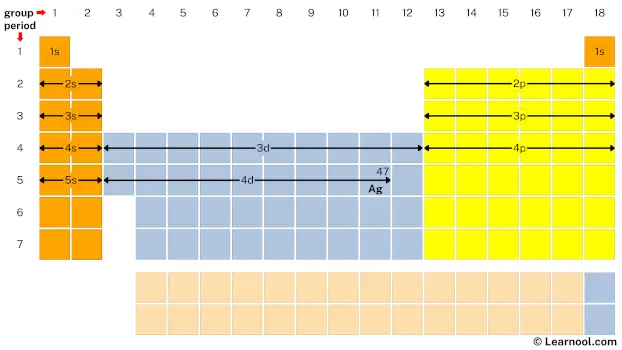
So the electron configuration of silver will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10.
Bohr model
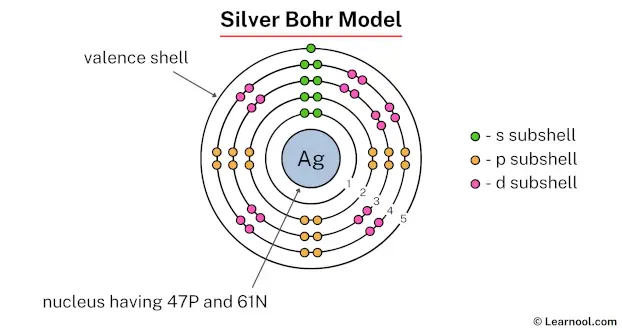
In the above image, 1 represents the 1st electron shell. Similarly, 2 represents the 2nd electron shell, 3 represents the 3rd electron shell, 4 represents the 4th electron shell, and 5 represents the 5th electron shell.
The 1st electron shell contains 1s subshell, the 2nd electron shell contains 2s and 2p subshells, the 3rd electron shell contains 3s, 3p, and 3d subshells, the 4th electron shell contains 4s, 4p, and 4d subshells, and the 5th electron shell contains 5s subshell.
We know that each s subshell can hold maximum 2 electrons, each p subshell can hold maximum 6 electrons, each d subshell can hold maximum 10 electrons, and each f subshell can hold maximum 14 electrons.
Also, we have to make sure that the electron configuration will match the order of aufbau principle (i.e., the 1s subshell is filled first and then 2s, 2p, 3s… and so on).
So the electron configuration of silver will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10.
Where,
1s2 indicates that the 1s subshell has 2 electrons
2s2 indicates that the 2s subshell has 2 electrons
2p6 indicates that the 2p subshell has 6 electrons
3s2 indicates that the 3s subshell has 2 electrons
3p6 indicates that the 3p subshell has 6 electrons
4s2 indicates that the 4s subshell has 2 electrons
3d10 indicates that the 3d subshell has 10 electrons
4p6 indicates that the 4p subshell has 6 electrons
5s1 indicates that the 5s subshell has 1 electron
4d10 indicates that the 4d subshell has 10 electrons
Learn how to draw: Silver Bohr model
Orbital diagram

The above orbital diagram shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p subshell has 6 electrons, the 4s subshell has 2 electrons, the 3d subshell has 10 electrons, the 4p subshell has 6 electrons, the 5s subshell has 1 electron, and the 4d subshell has 10 electrons.
So the electron configuration of strontium will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10.
Next: Cadmium electron configuration
Related
More topics
External links
- https://socratic.org/questions/what-is-the-electron-configuration-of-ag
- https://valenceelectrons.com/silver-electron-configuration/
- https://materials.gelsonluz.com/2019/08/electron-configuration-of-silver-ag.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
