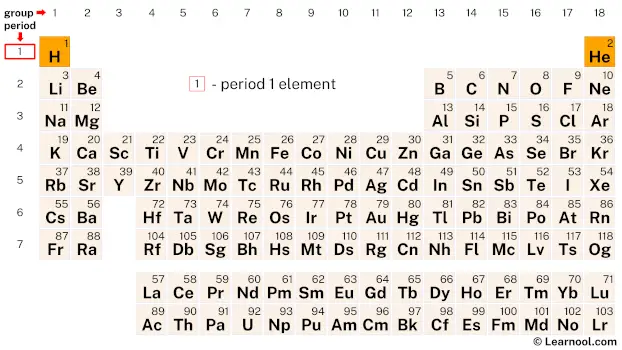
Period 1 elements refer to the chemical elements located in the first row or period of the periodic table. This group of elements includes hydrogen (H) and helium (He), the two most abundant elements in the universe. Despite their small size and low atomic numbers, period 1 elements exhibit unique chemical and physical properties that make them important building blocks for more complex compounds and materials. One of their notable characteristics is that period 1 elements obey the duet rule, which means they require only two electrons to complete their valence shell.
Hydrogen, the lightest and simplest element, is highly reactive and can form a variety of compounds with other elements, including water (H2O) and methane (CH4). It is also used as a fuel source in hydrogen fuel cells and as a raw material for the production of ammonia, a key component in fertilizers.
Helium, on the other hand, is inert and does not readily form compounds with other elements. It is best known for its use in balloons, blimps, and other lighter-than-air aircraft due to its low density and non-flammable properties.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| 1 | – period 1 element |
Period 1 of the periodic table consists of only two elements: hydrogen and helium.
Element information
Hydrogen

Hydrogen is the first and lightest element in the periodic table, with an atomic number of 1. It is a colorless, odorless, and highly flammable gas that is the most abundant element in the universe. Hydrogen is also the most reactive element and readily forms compounds with most other elements.
Hydrogen has many important uses. It is used as a fuel source in hydrogen fuel cells, which convert the energy from hydrogen into electricity. It is also used in the production of ammonia, which is a key component in fertilizers, and in the production of other chemicals and materials, such as methanol and polymers.
Helium
Helium is the second element in the periodic table, with an atomic number of 2. It is a colorless, odorless, and tasteless gas that is the second most abundant element in the universe, after hydrogen. Unlike hydrogen, helium is inert and does not readily form compounds with other elements.
Helium has several unique properties that make it useful in a variety of applications. Its low density and non-flammable properties make it an ideal choice for filling balloons, blimps, and other lighter-than-air aircraft. It is also used in cryogenics, as it has the lowest boiling point of any element, and in welding and other industrial processes, where its inert properties make it an effective shield gas.
Properties
Period 1 elements have unique properties that distinguish them from other elements in the periodic table. These include small atomic radii, low densities, and low melting and boiling points. Hydrogen, the first element in the group, is a gas at room temperature, while helium, the second element, is a gas at all temperatures except under extremely low temperatures, where it becomes a liquid. Although period 1 elements have low natural abundance, they are highly important in various applications, such as hydrogen in fuel cells and helium in cryogenics.
Period 1 elements vs. others
Period 1 elements differ from the other elements in the periodic table in several ways. One of the key differences is their electronic configuration. While the elements in the other periods all have electrons occupying at least one p-orbital, period 1 elements have only s-orbitals occupied. Period 1 elements are generally more reactive than the elements in the other periods due to their small size and low ionization energies. This reactivity is particularly evident in the case of hydrogen, which can readily react with a wide range of other elements to form a variety of compounds. However, despite their unique properties, period 1 elements have limited industrial and commercial applications due to their low natural abundance and difficult extraction.
Related
More topics
- Period 1 element
- Period 2 element
- Period 3 element
- Period 4 element
- Period 5 element
- Period 6 element
- Period 7 element
External links
- Period 1 element – Wikipedia
- Period 1 element Facts for Kids – Kids encyclopedia facts
- Period 1 element – Chem Europe
- Period 1 element – wikidoc
- Period 1 Element – Academic Accelerator
- Period 1 element – Wikiwand
- Period 1 element – Academic Kids
- Why are there only two elements in the first period of the periodic table? – Brainly
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
