
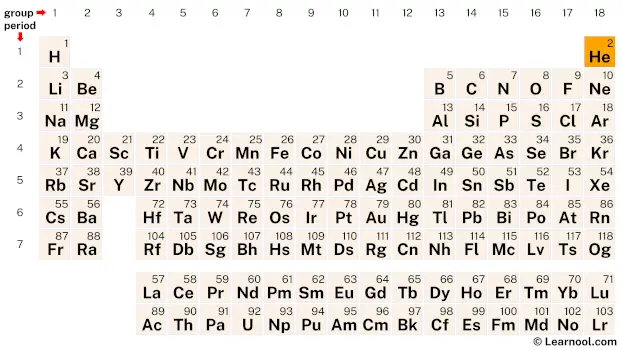
Helium (He) is a chemical element of the periodic table, located in the group 18 and the period 1, and is having the atomic number 2. It is a colorless, odorless, tasteless, non-toxic gas, whose name comes from the Greek word “helios”, which means sun. It is the first element in the noble gas group. It is the second lightest element, after the hydrogen element.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – s block |
Helium is an s-block element, found in the eighteenth column and the first row of the periodic table. It has the atomic number 2 and is denoted by the symbol He.
Element information
 |
|
 |
|
| Origin of name | Greek word “helios” (which means sun) |
| Symbol | He |
| Atomic number (Z) | 2 |
| Atomic mass | 4.002602 u |
| Block | s-block |
| Group | 18 |
| Period | 1 |
| Classification | Noble gas |
| Covalent radius | 28 pm |
| Van der Waals radius | 140 pm |
| Melting point | -272.20 ℃, -457.96 ℉, 0.95 K |
| Boiling point | -268.928 ℃, -452.070 ℉, 4.222 K |
| Electron configuration | 1s2 |
| Electrons per shell | 2 |
| Learn how to draw: Helium Bohr model | |
| Crystal structure | Hexagonal close-packed (hcp) |
| Phase at r.t | Gas |
| Density near r.t | 0.1786 g/L |
| Main isotopes | Helium-3, Helium-4 |
| Natural occurrence | Primordial |
| Oxidation state | 0 |
| Protons Neutrons Electrons |
2 2 2 |
| Learn how to find: Helium protons neutrons electrons | |
| Valence electrons | 2 |
| Learn how to find: Helium valence electrons | |
| CAS number | 7440-59-7 |
| Discovered by | Pierre Janssen and Norman Lockyer in 1868 |
History
Helium was first discovered in 1868 by French astronomer Jules Janssen and British astronomer Joseph Norman Lockyer while observing a solar eclipse. They detected a yellow spectral line in the light spectrum of the sun, which they identified as the element helium.
The name helium is derived from the Greek word ‘helios’, meaning sun. After its discovery, helium was initially thought to exist only in the sun’s atmosphere. However, in 1895, Scottish chemist Sir William Ramsay and English chemist Morris Travers were able to isolate the element on Earth for the first time by treating the mineral cleveite with mineral acids.
Since then, helium has been found to be a widespread element in the universe, being the second most abundant element in the observable universe, after hydrogen. It is mostly produced by nuclear fusion in stars and is found in large quantities in natural gas fields, where it is extracted through liquefaction and fractional distillation.
In 1903, helium was used for the first time in an airship to lift a payload. This led to its use in blimps and later in balloons for various purposes, including military observation and meteorology. Helium’s low boiling point and non-reactive nature also make it a useful coolant for nuclear reactors and MRI machines.
Today, helium is an important industrial gas with a variety of applications, such as in welding, as a protective gas in arc welding, in gas chromatography, and as a coolant for space and nuclear applications. It is also used in cryogenics to cool materials and equipment to very low temperatures, and in the manufacturing of semiconductors and fiber optic cables.
Occurrence
Helium is the second lightest element and is the second most abundant element in the observable universe, after hydrogen. It is a noble gas and therefore, is chemically inert, meaning it does not react with other elements. Helium is formed by the natural radioactive decay of heavier elements, such as uranium and thorium, present in rocks and soils. It is also produced by fusion reactions in stars.
In the Earth’s atmosphere, helium is present at a concentration of about 5.2 parts per million by volume (ppmv). However, helium is relatively rare on Earth since it is light enough to escape the Earth’s gravitational pull and enter the atmosphere of space. As a result, helium is usually extracted from natural gas wells, where it is produced as a byproduct of the decay of uranium and thorium.
Production
The production of helium involves the extraction of helium from natural gas wells. Helium is usually present in natural gas deposits in small amounts, ranging from less than 1% to as much as 7%. The extraction of helium involves separating it from other gases, such as methane, using various methods such as liquefaction and adsorption.
One of the main sources of helium is the natural gas fields in the United States, particularly in Texas, Oklahoma, and Kansas. These gas fields contain high concentrations of helium, which are extracted and purified using cryogenic distillation. In this process, the natural gas is cooled to very low temperatures, causing the helium to liquefy and separate from the other gases.
Another method for producing helium is through the separation of helium from air. This method is less efficient than the extraction of helium from natural gas and is usually only used in certain specialized applications, such as for medical and scientific research. In this process, air is compressed and cooled to very low temperatures, causing the helium to liquefy and separate from the other gases. The resulting helium is then purified and stored for use.
Properties
Physical properties
Helium is a colorless, odorless, and tasteless gas.
It is the second lightest element and has the lowest boiling point of all elements.
It is insoluble in water and other liquids.
It has a very low density and is considered a noble gas.
Chemical properties
Helium is a noble gas and therefore has very low chemical reactivity.
It has a complete outer shell of electrons, making it very stable and unreactive.
It does not readily form compounds with other elements.
Nuclear properties
Helium has the highest binding energy per nucleon of all elements, making it the most stable element.
It has two protons and two neutrons in its nucleus, giving it a total of four nucleons.
3He and 4He are the two stable isotopes of helium.
Other properties
Helium has a very low melting point and is commonly used as a cooling agent for superconductors and other low-temperature applications.
It is also used in gas chromatography and as a lifting gas for balloons and airships.
Applications
Balloons and blimps
Helium is widely used as a lifting gas in balloons, blimps, and airships. Due to its low density, it provides buoyancy to the vehicle, allowing it to float in the air.
Welding
Helium is used as a shielding gas in welding. When used with a welding arc, it helps to protect the weld from oxidation and prevents contamination from the surrounding air.
Cooling agent
Helium is used as a cooling agent in many applications, including nuclear reactors, MRI machines, and cooling of electronic components such as computer chips.
Leak detection
Helium is an inert gas and is used as a tracer gas in leak detection. It is often used to test the integrity of pipelines and other sealed systems.
Breathing gas
Helium is used as a breathing gas in deep-sea diving and other high-pressure environments. Due to its low density, it can reduce the risk of decompression sickness.
Gas chromatography
Helium is often used as a carrier gas in gas chromatography. Its low viscosity and high thermal conductivity make it ideal for separating and analyzing different compounds in a mixture.
Astronomy
Helium is a major component of stars and is used in astronomy to study the properties of stars and galaxies. It is also used as a coolant in space telescopes such as the Hubble Space Telescope.
Interesting facts
Helium is the second lightest element in the universe, after hydrogen, and is the second most abundant element in the observable universe, being present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined.
Helium is the only element that was first discovered in outer space before it was discovered on Earth. It was first detected in the spectrum of the Sun’s light by French astronomer Jules Janssen and British astronomer Joseph Norman Lockyer in 1868.
Helium has the lowest boiling point of any element and cannot be solidified at standard pressure, even at absolute zero. It remains a gas unless it is subjected to extreme pressure.
Helium is the only element that cannot be solidified by lowering the temperature. It remains a gas until it is subjected to extreme pressure, which causes it to become a liquid.
Helium is widely used as a coolant in cryogenics, particularly in the cooling of superconducting magnets, such as those used in MRI machines.
Helium is also used in gas chromatography and as a carrier gas in analytical chemistry.
Helium is used as a lifting gas for balloons, blimps, and airships due to its low density.
The United States is the world’s largest producer and consumer of helium, and the Federal Helium Reserve in Amarillo, Texas, is the world’s largest helium storage facility.
Liquid helium is used to cool the sensors of the Hubble Space Telescope to near absolute zero, allowing for greater precision in its observations.
Helium has two stable isotopes, helium-3 and helium-4, with helium-4 being the most common. Helium-3 is much rarer and has potential applications in nuclear energy and medical imaging.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/2/helium
- https://www.britannica.com/science/helium-chemical-element
- https://en.wikipedia.org/wiki/Helium
- https://education.jlab.org/itselemental/ele002.html
- https://pubchem.ncbi.nlm.nih.gov/element/Helium
- https://www.chemicool.com/elements/helium.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
