
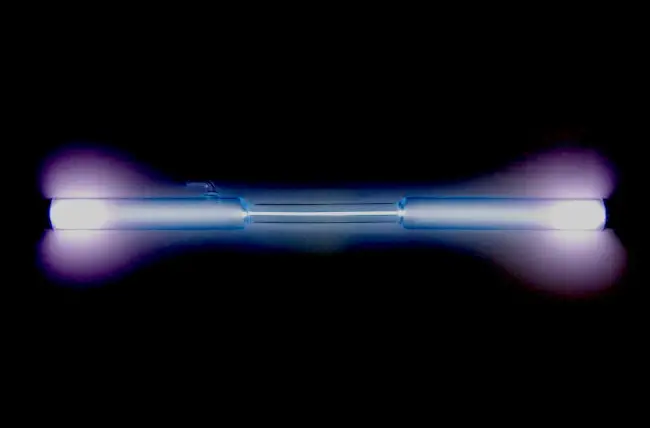
Xenon (Xe) is a chemical element of the periodic table, located in the group 18 and the period 5, and has the atomic number 54. It is a colorless, odorless, tasteless gas, whose name comes from the Greek word “xenos”, which means stranger. It is a member of the noble gas group.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – p block |
Xenon is a p-block element, found in the eighteenth column and the fifth row of the periodic table. It has the atomic number 54 and is denoted by the symbol Xe.
Element information
 |
|
 |
|
| Origin of name | Greek word “xenos” (which means stranger) |
| Symbol | Xe |
| Atomic number (Z) | 54 |
| Atomic mass | 131.293 u |
| Block | p-block |
| Group | 18 |
| Period | 5 |
| Classification | Noble gas |
| Covalent radius | 140±9 pm |
| Van der Waals radius | 216 pm |
| Melting point | -111.75 ℃, -169.15 ℉, 161.40 K |
| Boiling point | -108.099 ℃, -162.578 ℉, 165.051 K |
| Electron configuration | [Kr] 4d10 5s2 5p6 |
| Electrons per shell | 2, 8, 18, 18, 8 |
| Learn how to draw: Xenon Bohr model | |
| Crystal structure | Face-centered cubic (fcc) |
| Phase at r.t | Gas |
| Density near r.t | 5.894 g/L |
| Main isotopes | Xenon-126, Xenon-128, Xenon-129, Xenon-130, Xenon-131, Xenon-132, Xenon-134 |
| Natural occurrence | Primordial |
| Oxidation state | 0 |
| Electronegativity (Pauling scale) | 2.60 |
| Protons Neutrons Electrons |
54 77 54 |
| Learn how to find: Xenon protons neutrons electrons | |
| Valence electrons | 8 |
| CAS number | 7440-63-3 |
| Discovered by | William Ramsay and Morris Travers in 1898 |
History
Xenon was discovered by the Scottish chemist Sir William Ramsay and English chemist Morris William Travers in 1898. They isolated the gas by fractional distillation of liquid air. Ramsay named the gas “xenon,” which means “stranger” or “foreigner” in Greek, due to its rarity and unique properties.
After the discoveries of helium, neon, argon, and krypton, xenon became the fifth noble gas to be discovered. Its discovery not only challenged the periodic law of the elements but also opened up new avenues for further discoveries of noble gases.
In the early 20th century, xenon was primarily used in scientific research and as a medical anesthetic. However, its use was limited due to its high cost and difficulty in obtaining large quantities. It was not until the 1960s that advances in gas liquefaction technology allowed for the commercial production of xenon.
Today, xenon is used in a variety of applications, including lighting, nuclear energy, aerospace, and medical imaging. Its unique properties, such as its high density and low reactivity, make it a valuable element in these industries.
Occurrence and production
Xenon is a rare and inert gas that occurs in trace amounts in Earth’s atmosphere, with a volume fraction of approximately 1 part per 11.5 million. It is also found in gases emitted from some mineral springs. The total mass of xenon in Earth’s atmosphere is estimated to be around 2.03 gigatonnes, which accounts for only a small fraction of the atmosphere’s composition. Xenon is relatively rare in the Solar System, being present in asteroids, comets, and the atmosphere of planet Jupiter.
The commercial production of xenon is achieved through the separation of air into oxygen and nitrogen by fractional distillation in a double-column plant. The liquid oxygen produced in this process contains small amounts of krypton and xenon, which can be extracted by further fractional distillation. The liquid oxygen may be enriched to contain 0.1 to 0.2% of a krypton/xenon mixture, which is then separated into krypton and xenon by distillation. This process is both energy-intensive and costly, as xenon is scarce compared to other noble gases like argon and neon.
Worldwide production of xenon was estimated at 5,000 to 7,000 cubic meters in 1998. Due to its scarcity, xenon is much more expensive than other noble gases. For example, in 1999, approximate prices for the purchase of small quantities in Europe were 10 €/L for xenon, 1 €/L for krypton, and 0.20 €/L for neon. The commercial use of xenon is primarily in lighting and medical applications.
Properties
Physical properties
Xenon is a colorless, odorless, and tasteless gas.
It is a dense gas, with a density of 5.89 g/L at standard temperature and pressure.
Xenon is a noble gas and has a very low reactivity towards other elements.
Chemical properties
Xenon is a chemically inert gas and does not form chemical compounds with other elements under normal conditions.
However, under extreme conditions such as high pressure and high temperature, xenon can form some chemical compounds like xenon hexafluoride (XeF6) and xenon tetrafluoride (XeF4).
Xenon is capable of forming weak van der Waals bonds with other elements, and this property has some applications in organic chemistry.
Isotopes
Xenon has seven stable isotopes and over 30 artificial unstable isotopes.
Xenon-129 is the most abundant stable isotope, making up about 26.4% of natural xenon.
Xenon-129 has various applications, including its use in environmental studies and geological dating, as well as in medical imaging techniques such as MRI.
Other properties
Xenon is a good insulator of heat and electricity, and this property has some applications in lighting technology.
Xenon has potential applications in space exploration as a propellant for ion engines due to its high atomic mass and low ionization potential.
Applications
Lighting
Xenon is used in high-intensity discharge lamps for various purposes including automotive headlights, photographic and movie lighting, and searchlights. These lamps are preferred due to their high efficiency, long lifespan, and color rendering ability.
Medicine
Xenon has several medical uses. It can be used as an anesthetic for surgeries, as it is non-flammable, non-toxic, and has a low solubility in blood, which makes it easy to control its concentration.
Additionally, xenon has been shown to have neuroprotective properties and may be useful in the treatment of stroke, traumatic brain injury, and other neurological disorders.
Aerospace
Xenon is used in ion thrusters for spacecraft propulsion. Ion thrusters generate small amounts of thrust but can operate for long periods of time, making them ideal for deep space missions.
Nuclear energy
Xenon is produced during nuclear fission and can be used to analyze and monitor nuclear reactions. It can also be used as a coolant in nuclear reactors.
Other uses
Xenon is used in certain types of lasers, as a detector in particle physics experiments, and as a tracer gas for leak detection in air conditioning and refrigeration systems.
Interesting facts
Xenon is a noble gas and is considered to be the heaviest of all the stable noble gases.
The name “xenon” comes from the Greek word “xenos,” meaning “stranger” or “foreigner.”
Xenon is used in lighting technology, such as high-intensity discharge lamps (HID), where it produces a bright white light that closely resembles daylight.
Xenon is used in medical imaging, such as MRI and CT scans, as a contrast agent.
Xenon is also used in the production of semiconductors, as a coolant for nuclear reactors, and in ion propulsion systems for spacecraft.
Xenon has seven stable isotopes, which is more than any other noble gas element, including krypton, which has only five stable isotopes.
Xenon is known to form compounds with highly electronegative elements like fluorine, but these are highly unstable and decompose quickly.
Xenon is an inert gas that is rare in the Earth’s atmosphere and has very low reactivity with the environment.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/54/xenon
- https://en.wikipedia.org/wiki/Xenon
- https://www.britannica.com/science/xenon
- https://pubchem.ncbi.nlm.nih.gov/element/Xenon
- https://www.chemicool.com/elements/xenon.html
- https://education.jlab.org/itselemental/ele054.html
- https://www.livescience.com/37504-facts-about-xenon.html
- https://sciencenotes.org/xenon-facts-and-uses-atomic-number-54-element-symbol-xe/
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
