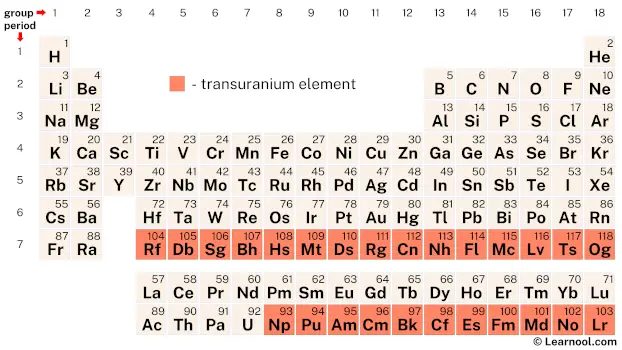
Transuranium elements, also known as transuranic elements, encompass a set of 26 chemical elements with atomic numbers greater than that of uranium (92). This set includes the first 11 elements beyond uranium, known as actinides, and the subsequent 15 elements, known as transactinides. With the exception of neptunium (93) and plutonium (94), all transuranium elements are synthetic, meaning they are not naturally found on Earth but are created artificially. These elements are highly unstable and undergo radioactive decay processes, transforming into other elements over time.
Transuranium elements, due to their highly unstable nature, are highly radioactive, and they emit various types of radiation as they undergo decay. These elements experience radioactive decay processes, such as alpha decay, beta decay, and in some cases, spontaneous fission, which leads to the transformation of the transuranium elements into other elements. These radioactive properties make them valuable for various applications, including medical diagnostics, nuclear power generation, and scientific research. However, the short half-lives and hazardous nature of transuranium elements pose significant challenges for their handling and containment. As a result of their synthetic nature, transuranium elements are produced in laboratories through nuclear reactions involving heavy elements as target materials, which makes them important subjects of study in the field of nuclear chemistry.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – transuranium element |
On the periodic table, there are a total of 26 chemical elements that fall under the category of transuranium elements. These elements have atomic numbers greater than that of uranium (92) and span from neptunium (93) to oganesson (118).
Related
More topics
- Transuranium element
- Superheavy element
- Rare-earth element
- Synthetic element
- Main-group element
- Inner transition metal
External links
- Transuranic Elements – Health Risks of Radon and Other Internally Deposited Alpha-Emitters – National Institutes of Health (.gov)
- Transuranium element – Wikipedia
- Transuranium element | Definition & Examples – Britannica
- Transuranic Element – Nuclear Regulatory Commission (.gov)
- The transuranic elements and the island of stability – The Royal Society
- 25.4: Transuranium Elements – Chemistry LibreTexts
- Transuranium Elements and the Physical Review – Physical Review Journals
- Transuranium Elements at Berkeley Lab – American Chemical Society
- Transuranium element – European Nuclear Society
- Do transuranic elements such as plutonium ever occur naturally? – Scientific American
- The Periodic Table and Transuranium Elements – American Chemical Society
- Transuranium element – Elements Wiki | Fandom
- The Transuranium Elements: From Neptunium and Plutonium to Element 112 – International Atomic Energy Agency
- Transuranium Elements: Definition & Production – Study.com
- Transuranium Element – an overview – ScienceDirect
- The Transuranium Elements – eScholarship
- ERIC – EJ321577 – The Transuranium Elements., Journal of Chemical Education, 1985 – Department of Education (.gov)
- Transuranium element – Chem Europe
- Transuranium Elements – Springer
- Transuranium Elements | Introduction to Chemistry – Course Hero
- What is transuranium element in the periodic table? – Quora
- Transuranium elements – The Free Dictionary
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
