
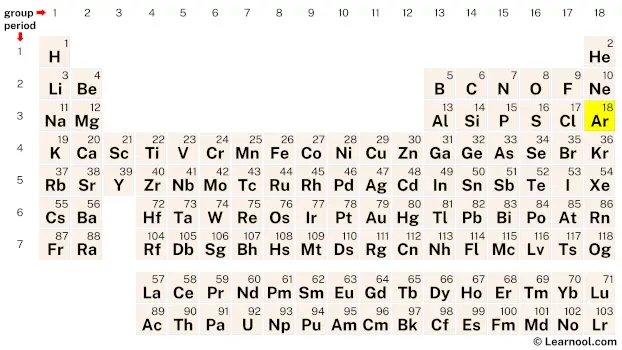
Argon (Ar) is a chemical element of the periodic table, located in the group 18 and the period 3, and is having the atomic number 18. It is a colorless, odorless, tasteless gas, whose name comes from the Greek word “argos”, which means lazy or inactive. It is a member of the noble gas group.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – p block |
Argon is a p-block element, situated in the eighteenth column and the third row of the periodic table, denoted by the atomic number 18 and chemical symbol Ar.
Element information
 |
|
 |
|
| Origin of name | Greek word “argos” (which means lazy or inactive) |
| Symbol | Ar |
| Atomic number (Z) | 18 |
| Atomic mass | 39.948 u |
| Block | p-block |
| Group | 18 |
| Period | 3 |
| Classification | Noble gas |
| Covalent radius | 106±10 pm |
| Van der Waals radius | 188 pm |
| Melting point | -189.34 ℃, -308.81 ℉, 83.81 K |
| Boiling point | -185.848 ℃, -302.526 ℉, 87.302 K |
| Electron configuration | [Ne] 3s2 3p6 |
| Learn how to write: Argon electron configuration | |
| Electrons per shell | 2, 8, 8 |
| Learn how to draw: Argon Bohr model | |
| Crystal structure | Face-centered cubic (fcc) |
| Phase at r.t | Gas |
| Density near r.t | 1.784 g/L |
| Main isotopes | Argon-36, Argon-38, Argon-40 |
| Natural occurrence | Primordial |
| Oxidation state | 0 |
| Protons Neutrons Electrons |
18 22 18 |
| Learn how to find: Argon protons neutrons electrons | |
| Valence electrons | 8 |
| Learn how to find: Argon valence electrons | |
| CAS number | 7440-37-1 |
| Discovered by | Lord Rayleigh and William Ramsay in 1894 |
History
Argon was discovered in 1894 by British scientists Sir William Ramsay and Lord Rayleigh. The two scientists were working independently on their research on air, and both noticed an unexpected gas that they could not identify. The gas was eventually identified as a new element and named “argon” from the Greek word “argos,” which means “inactive.”
Ramsay and Rayleigh found that argon is a component of the Earth’s atmosphere, comprising approximately 1% by volume. It is the third-most abundant gas in the atmosphere, after nitrogen and oxygen. They also discovered that argon is present in some minerals and in volcanic gases.
The discovery of argon marked a turning point in the field of chemistry, as it was the first time a noble gas was identified. This sparked interest in the study of other noble gases, and eventually led to the discovery of helium, neon, krypton, and xenon.
Today, argon is produced by the fractional distillation of liquid air. It is also produced as a byproduct of the production of other gases, such as oxygen and nitrogen. Argon is used in a variety of applications, including welding, lighting, and as a protective gas in the production of metals and semiconductors.
Occurrence and production
Argon is the third most abundant gas in the Earth’s atmosphere, accounting for about 0.934% of the air. It is also found in small amounts in some minerals, such as potassium feldspar and some rocks from the Earth’s mantle. Additionally, argon is found in some meteorites and in the gas surrounding some stars.
Argon is produced commercially by the distillation of liquid air. This process takes advantage of the fact that argon has a lower boiling point than other components of air, such as nitrogen and oxygen. The air is first compressed and cooled, causing it to liquefy. The liquid air is then allowed to slowly warm up, causing the components to vaporize at different temperatures. The argon is collected as it boils off, leaving behind the other gases. This process is repeated several times to produce a high purity of argon. Some other methods for producing argon include the combustion of magnesium in air and the decomposition of sodium nitride.
Properties
Physical properties
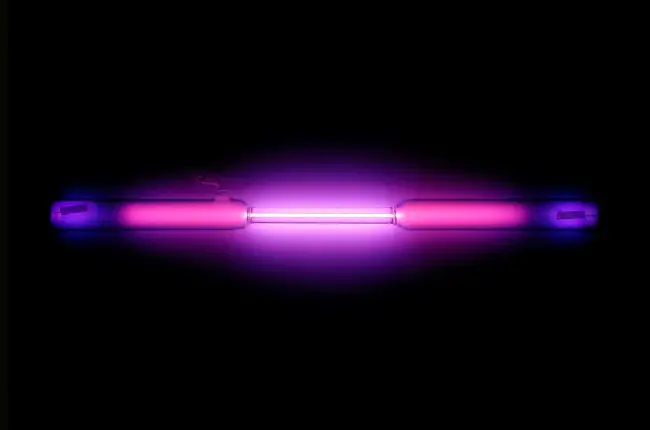
Argon is a colorless, odorless, and tasteless noble gas.
It is non-flammable and non-reactive under normal conditions.
Its boiling point is -185.848 ℃ and its melting point is -189.34 ℃, which are both lower than those of iron and copper.
Chemical properties
Argon is a noble gas and has a full valence shell of eight electrons, making it chemically inert.
It does not easily form compounds with other elements and is therefore considered to be non-reactive.
Isotopes
Argon has three stable isotopes: 36Ar, 38Ar, and 40Ar.
40Ar is the most abundant, accounting for over 99% of the argon found on Earth.
39Ar, a radioactive isotope, is used in dating rocks and minerals.
Other properties
Argon is denser than air and is commonly used as a shielding gas in welding and other high-temperature industrial processes.
It is also used in gas-filled electric light bulbs, as well as in gas lasers and plasma displays.
Applications
Argon is used as a shielding gas in welding processes, as it prevents oxidation and other reactions from occurring during the welding process.
Argon gas is commonly used in fluorescent lighting and other types of gas-discharge lamps.
Argon is used to preserve historical documents, old photographs, and other valuable items that are susceptible to damage from exposure to air.
Argon is sometimes used as a refrigerant in certain types of cooling systems.
Argon gas is sometimes used in medical applications, such as in surgeries where it is used to inflate the abdomen during laparoscopic procedures.
Argon gas is used in various scientific research applications, such as in gas chromatography and in experiments involving the study of gases.
Interesting facts
Argon is the third most common gas in the Earth’s atmosphere, after nitrogen and oxygen.
It was discovered in 1894 by the British scientist Lord Rayleigh and his assistant Sir William Ramsay.
The name “argon” comes from the Greek word “argos,” meaning “lazy” or “inactive,” which reflects its non-reactive nature.
Argon is often used in welding and metal fabrication because it helps to prevent oxidation and other chemical reactions that can weaken the metal.
It is also used in light bulbs to prevent the tungsten filament from oxidizing and burning out too quickly.
Argon has medical applications as well, such as being used in laser surgery and as a shielding gas during radioactive isotope production.
In 2000, scientists were able to produce the first ever molecular compound of argon by combining it with a fluorine compound.
Argon is used in the dating of rocks and minerals because it is a stable, inert gas that does not react with other elements, and can be trapped in rocks over long periods of time.
Argon has the lowest thermal conductivity of any known gas, making it a good insulator.
Argon is sometimes used in wine preservation to prevent oxidation and spoilage.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/18/argon
- https://en.wikipedia.org/wiki/Argon
- https://www.britannica.com/science/argon-chemical-element
- https://education.jlab.org/itselemental/ele018.html
- https://pubchem.ncbi.nlm.nih.gov/element/Argon
- https://chemistrytalk.org/argon-element/
- https://www.chemicool.com/elements/argon.html
- https://www.livescience.com/29023-argon.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
