
Potassium (K) is a chemical element of the periodic table, located in group 1 and period 4, having the atomic number 19. It is a soft, silvery white alkali metal whose name comes from the English word “potash”. In nature, potassium only exists in ionic salts. Naturally occurring potassium is made of three isotopes, of which 39K and 41K are stable.
The majority of potassium is found in igneous rocks, shale, and silt in minerals like muscovite and orthoclase feldspar that are insoluble in water. With water, it undergoes a very vigorous reaction that releases hydrogen and produces a potassium hydroxide (KOH) solution. And it produces potassium peroxide (K2O2) when it comes in contact with oxygen.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – s block |
Potassium is an s-block element, situated in the first column and the fourth row of the periodic table, denoted by the atomic number 19 and chemical symbol K.
Element information
| Potassium element | |
|---|---|
| Symbol | K |
| Atomic number (Z) | 19 |
| Standard atomic weight | 39.0983 |
| CAS number | 7440-09-7 |
| Origin of name | From the English word potash; symbol from Latin kalium |
| Group | 1 |
| Period | 4 |
| Block | s |
| Classification | Alkali metal |
| Electron configuration | [Ar] 4s1 |
| Electrons per shell | 2, 8, 8, 1 |
| Learn how to draw: Potassium Bohr model | |
| Valence electrons | 1 |
| Learn how to find: Potassium valence electrons | |
| Protons, neutrons, electrons | 19 protons, 20 neutrons, 19 electrons (for most common isotope: 39K) |
| Learn how to find: Potassium protons neutrons electrons | |
| Oxidation state(s) | +1 |
| Electronegativity (Pauling scale) | 0.82 |
| Atomic radius | 227 pm |
| Covalent radius | 203 pm |
| Van der Waals radius | 275 pm |
| Phase at room temperature | Solid |
| Crystal structure | Body-centered cubic (bcc) |
| Density near room temperature | 0.862 g/cm3 |
| Melting point | 63.5 ℃ |
| Boiling point | 759 ℃ |
| Main isotopes | 39K, 40K, 41K |
| Natural occurrence | Primordial; found in minerals like sylvite and carnallite |
| Discovered by | Humphry Davy, 1807 |
History
Potassium was first derived from potash, meaning plant ashes, from which it received its name. The English term first appeared in 1648 and is a borrowed translation of the Dutch word potaschen. The chemical symbol K derives from kalium, the Mediaeval Latin for potash, which may have evolved from the Arabic term qali, meaning alkali.[1] Potash and soda (sodium carbonate, Na2CO3) were both referred to as alkali by the Arabs, and there was no distinction between them from antiquity until the Middle Ages. Both substances were known as natron in Europe.

Martin Heinrich Klaproth was the first to differentiate between the two alkalis, suggesting the names kali for vegetable alkali and natron for mineral alkali.[2] Humphry Davy gave the element the name potassium, which he derived from the word potash after he separated the pure element using electrolysis in 1807.
The metals were initially referred to as métal de potasse and métal de soude, and later as Potassium and Sodium by Gay-Lussac and Thénard, who also studied the alkalis. Davy’s research findings were published in German by Ludwig Wilhelm Gilbert. He undoubtedly used Klaproth’s suggestion from 1797.
Gehlen afterwards suggested Kalin(um) and Natrin or Natrinmetall in 1808. Berzelius shortened Potassium and Sodium as Po and So in 1813. Despite a list of names in many languages, Gilbert and Klaproth’s names Natrium and Kalium were eventually chosen by Germanic countries, while Davy and Gay-Lussac/Thénard’s names Sodium and Potassium were accepted by English and French-speaking countries.
Occurrence
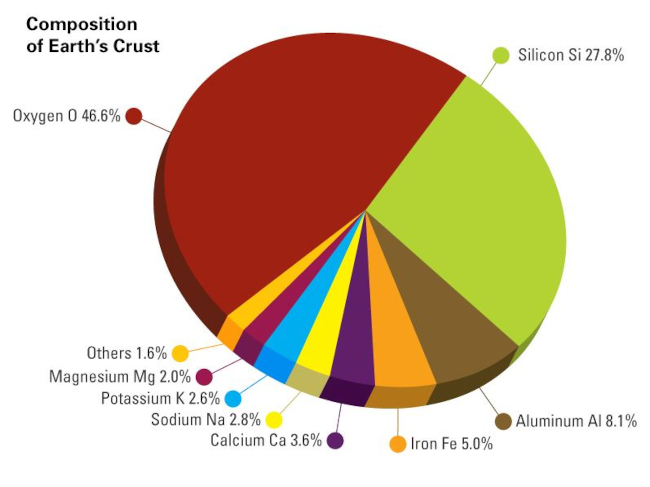
Potassium is not found in its elemental form in nature because it reacts so easily with water. Because of this, the majority of potassium is found in the Earth’s crust as clays and feldspars. It is the seventh most abundant element in the Earth’s crust, making up around 2.6% of its weight. Numerous additional saline bodies of water are rich in potassium, with the Dead Sea having an estimated potassium chloride level of 1.7%. All fruits, vegetables, meat, and fish contain potassium. Potassium is abundant in leafy greens, beans, nuts, dairy products, milk, chocolate, bananas, and avocados.
Production
Most commercial potassium compounds are produced by electrolysis from soluble potassium compounds, such as carnallite, sylvite, polyhalite, and langbeinite, which are discovered in old lakes and seabeds. In 2021, it was predicted that the world would produce about 71.9 million tonnes of potash. Canada is the world’s largest potash producer, accounting for 31% of world production in 2021.[3] About 70% of the potash produced worldwide in 2021 was produced by three nations Belarus, Russia, and Canada.
Metallic potassium and sodium-potassium alloys (NaK) are produced by reacting high temperature sodium at atmospheric pressure with molten potassium chloride.[4]
Na + KCl → NaCl + K
An equilibrium vapor of sodium and potassium is produced when molten potassium chloride is added to a tightly packed column and brought into contact with rising sodium vapors in a reaction zone. The lighter boiling potassium is separated to the required degree of purity using a fractionating column located above the reaction zone. The generated sodium chloride is continually removed from beneath the reaction zone.
Properties

Potassium is easily sliced with a knife. It is silvery when first cut, but as soon as it comes into contact with oxygen, it starts to tarnish and turn gray.
It is one of the alkali metals in the periodic table that possesses a single valence electron in the outer electron shell.
It has a low melting point and is a good conductor of heat and electricity.
Pure potassium is a highly reactive metal that explodes with a purple flame when it comes in contact with water. Hence for safety reasons, this metal is typically stored under mineral oil.
Applications
Fertilizers use potassium. It plays a significant role in boosting crop yields and overall crop quality, as well as strengthening plants’ defenses against disease.
It is combined with sodium to form an alloy that acts as a liquid metallic heat-transfer medium.
Potassium is used as a bleaching agent in the textile industry and also used in the tanning of leather.
Potassium is used as a flavor enhancer in food, and it is also used as a preservative to prevent food from spoiling.
Potassium is used as a medication to prevent or treat hypokalemia (low blood levels of potassium).
Potassium is used as an oxidizing agent in gunpowder. Also used in explosives, fireworks, and matches.
Potassium is used in color TV tubes and fluorescent lamps. It is used as a drying agent in laboratories. And is used in the manufacture of glass, liquid soap, and detergent.
Many different kinds of magnetometers utilize metallic potassium.
Interesting facts
Potassium is the seventh most abundant element in the human body.
Potassium was the first metal discovered by electrolysis.
Potassium is less dense than water, so it floats on water.

Potassium produces a violet or light purple flame, popularly known as a lilac flame.
Potassium is the second least dense metal after lithium.
Related
More elements
References
1. Atomic Weight of Potassium – CIAAW
2. Kalium (Potassium) – Elementymology & Elements Multidict
3. Potash facts
4. The Manufacture of Potassium and NaK – ACS Publications
External links
- https://www.rsc.org/periodic-table/element/19/potassium
- https://www.britannica.com/science/potassium
- https://en.wikipedia.org/wiki/Potassium
- https://pubchem.ncbi.nlm.nih.gov/element/Potassium
- https://chemistrytalk.org/potassium-element/
- https://www.chemicool.com/elements/potassium.html
- https://www.ducksters.com/science/chemistry/potassium.php
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
