
Strontium (Sr) is a chemical element of the periodic table, located in the group 2 and the period 5, and has the atomic number 38. It is a soft, silvery-white yellowish alkaline earth metal, whose name comes from Strontian, a small town in Scotland. It is a highly reactive metal that reacts with both air and water.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – s block |
Strontium is an s-block element, situated in the second column and the fifth row of the periodic table. Its atomic number is 38 and its symbol is Sr.
Element information
| Strontium element | |
|---|---|
| Symbol | Sr |
| Atomic number (Z) | 38 |
| Standard atomic weight | 87.62 |
| CAS number | 7440-24-6 |
| Origin of name | From Strontian, a village in Scotland |
| Group | 2 |
| Period | 5 |
| Block | s |
| Classification | Alkaline earth metal |
| Electron configuration | [Kr] 5s2 |
| Learn how to write: Strontium electron configuration | |
| Electrons per shell | 2, 8, 18, 8, 2 |
| Learn how to draw: Strontium Bohr model | |
| Valence electrons | 2 |
| Learn how to find: Strontium valence electrons | |
| Protons, neutrons, electrons | 38 protons, 50 neutrons, 38 electrons (for most common isotope: 88Sr) |
| Learn how to find: Strontium protons neutrons electrons | |
| Oxidation state(s) | +2 |
| Electronegativity (Pauling scale) | 0.95 |
| Atomic radius | 215 pm |
| Covalent radius | 195 pm |
| Van der Waals radius | 249 pm |
| Phase at room temperature | Solid |
| Crystal structure | Face-centered cubic (fcc) |
| Density near room temperature | 2.64 g/cm3 |
| Melting point | 777 °C |
| Boiling point | 1,382 °C |
| Main isotopes | 84Sr, 86Sr, 87Sr, 88Sr |
| Natural occurrence | Primordial; found in celestite and strontianite |
| Discovered by | William Cruickshank, 1790 (as a mineral); isolated by Humphry Davy, 1808 |
History

In 1790, Adair Crawford and William Cruickshank, two Scottish chemists, discovered strontium while analyzing samples of the mineral strontianite. They found that the mineral contained a new element, which they named strontium after the Scottish village of Strontian, where the mineral was first discovered.
Several years later, in 1808, Humphry Davy, an English chemist, was able to isolate pure strontium metal through electrolysis. He used a process in which an electric current was passed through a molten salt of strontium chloride. This process led to the discovery of the element’s metallic properties, as well as its highly reactive nature, especially when it comes into contact with water.
During the early 20th century, strontium became popular for its use in pyrotechnics, particularly in flares and fireworks, due to its brilliant red flame color. The element’s ability to produce bright colors also led to its use in color television tubes, where it was used to create a bright red color. Today, strontium is still used in pyrotechnics as well as an additive in some alloys, including aluminum alloys used in the aerospace industry, due to its ability to improve their strength and durability.
Occurrence and production
Strontium is the fifteenth most abundant element in the Earth’s crust, occurring at an average concentration of about 0.034%. It is found in minerals such as strontianite (SrCO3) and celestine (SrSO4), which are the primary sources of strontium. Strontium is also present in seawater, where its concentration is about 8 milligrams per liter.
In terms of production, the most common method for obtaining strontium metal is through the reduction of strontium oxide (SrO) with aluminum or other reducing agents. This process is carried out in a vacuum or inert atmosphere to prevent the formation of strontium oxide. The resulting strontium metal is then purified through distillation or electrolysis. Strontium compounds are also produced commercially by reacting strontium carbonate with various acids or other compounds.
Properties
Strontium has a silvery-white appearance, and is soft and malleable.
Strontium is a reactive metal that readily oxidizes in air and reacts violently with water.
It has a melting point of 777 ℃ and a boiling point of 1377 ℃.
Strontium is an alkaline earth metal and is located in Group 2 of the periodic table.
It has four stable isotopes and 15 known radioactive isotopes.
Strontium has a density of 2.64 g/cm3, which is lower than that of calcium, another alkaline earth metal.
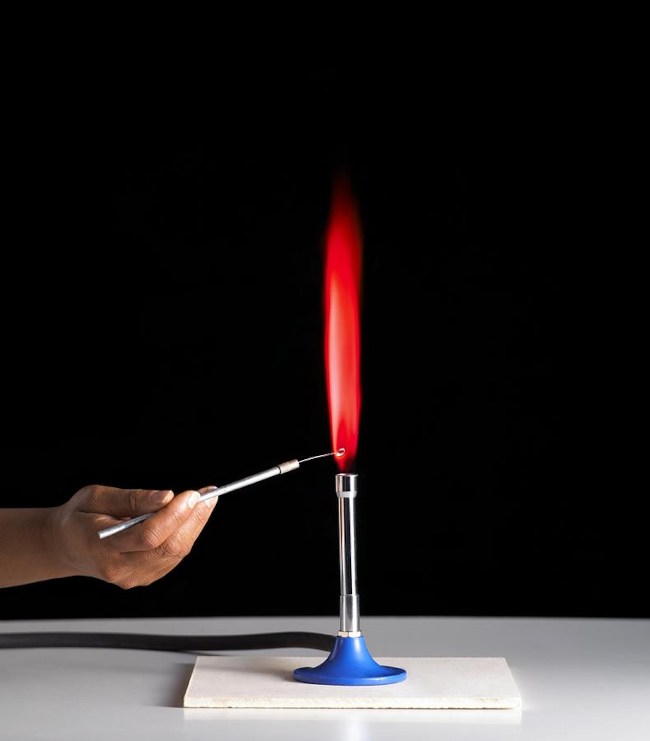
When burned, strontium produces a bright red flame, which is used in fireworks and flares.
Strontium ions can replace calcium ions in biological processes, affecting the uptake of calcium in bones and teeth.
Strontium compounds are used in the production of ferrite magnets, pyrotechnics, and flares, as well as in refining zinc and producing other metals.
Applications
Strontium compounds are used to produce red-colored flames in fireworks, due to the bright red light they emit when heated.
Strontium ranelate is a drug used to treat osteoporosis, as it can increase bone density and reduce the risk of fractures.
Strontium ferrite is a type of ceramic magnet that is used in various applications such as electric motors, magnetic bearings, and magnetic separations.
Strontium oxide is used as a flux in the production of special glasses for color television picture tubes.
Strontium-90, a radioactive isotope of strontium, has been used in radiography for medical imaging and industrial non-destructive testing.
Strontium chloride is used in some toothpaste formulations to help relieve tooth sensitivity by blocking the transmission of pain signals.
Strontium chromate is a yellow pigment used in some paints and coatings for its corrosion-resistant properties.
Interesting facts
Strontium is named after the Scottish village of Strontian where the mineral strontianite was first discovered.
Strontium is highly reactive and will ignite spontaneously in air, which makes it a useful addition to flares and fireworks.
In the early 20th century, strontium was used in toothpaste to help strengthen teeth, but it was later found to be harmful if ingested in large amounts.
Strontium has four stable isotopes and over 30 unstable isotopes, many of which are used in medical applications such as cancer treatment.
Strontium has been found in the bones of dinosaurs, and its presence in bones can be used to determine the age of fossils.
The strontium clock is a proposed method for measuring time in a way that is more accurate than atomic clocks. It is based on the decay rate of radioactive strontium isotopes.
Strontium has been detected in the atmosphere of stars and is used by astronomers to study the chemical composition of distant celestial bodies.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/38/strontium
- https://en.wikipedia.org/wiki/Strontium
- https://www.britannica.com/science/strontium
- https://pubchem.ncbi.nlm.nih.gov/element/Strontium
- https://www.livescience.com/34522-strontium.html
- https://www.chemicool.com/elements/strontium.html
- https://study.com/learn/lesson/stronium-facts-uses-properties-element.html
- https://www.radiochemistry.org/periodictable/elements/38.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
