
Lithium, having the symbol Li and atomic number 3, is a chemical element. It is a soft, silvery-white alkali metal that is located in group 1 of the periodic table, with helium and beryllium as its neighbors. Lithium is the lightest solid element and has a density about half that of water. Its name comes from the Greek word “lithos,” meaning stone, which refers to its discovery from a mineral source.
Lithium was discovered in 1817 by Swedish chemist Johan August Arfwedson while analyzing the mineral petalite. It was later isolated in its elemental form by William Thomas Brande in 1821 through the electrolysis of lithium oxide, a process that had previously been used by Humphry Davy to isolate other alkali metals such as sodium and potassium. Lithium is highly reactive and flammable, and it easily reacts with water to produce hydrogen gas. It has a low melting point and boiling point, making it useful for various applications in industry and scientific research.
Lithium has numerous applications in various fields, including energy storage, pharmaceuticals, and ceramics. One of its most well-known uses is in rechargeable lithium-ion batteries, which power many electronic devices such as smartphones and laptops. Lithium is also used in mood stabilizers for the treatment of bipolar disorder, and as an alloying agent in the production of lightweight metals like aluminum, commonly used in the aerospace industry.
Lithium has two naturally occurring stable isotopes, lithium-6 and lithium-7, with natural abundances of 7.59% and 92.41%, respectively. Lithium compounds have various industrial applications. For example, lithium hydroxide is used in air purification and in the production of greases and lubricants. Lithium chloride, on the other hand, is commonly used as a desiccant. Lithium is also used as a coolant in nuclear reactors due to its high thermal conductivity and low atomic weight.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – s block |
On the periodic table, lithium is located in the first column and second row, belongs to the alkali metal group, and is classified as an s-block element.
Element information
| Lithium element | |
|---|---|
| Symbol | Li |
| Atomic number (Z) | 3 |
| Standard atomic weight | 6.941 |
| CAS number | 7439-93-2 |
| Origin of name | From the Greek lithos, meaning “stone” |
| Group | 1 |
| Period | 2 |
| Block | s |
| Classification | Alkali metal |
| Electron configuration | 1s2 2s1 |
| Electrons per shell | 2, 1 |
| Learn how to draw: Lithium Bohr model | |
| Valence electrons | 1 |
| Learn how to find: Lithium valence electrons | |
| Protons, neutrons, electrons | 3 protons, 4 neutrons, 3 electrons (for most common isotope: 7Li) |
| Learn how to find: Lithium protons neutrons electrons | |
| Oxidation state(s) | +1 |
| Electronegativity (Pauling scale) | 0.98 |
| Atomic radius | 152 pm |
| Covalent radius | 128 pm |
| Van der Waals radius | 182 pm |
| Phase at room temperature | Solid |
| Crystal structure | Body-centered cubic (bcc) |
| Density near room temperature | 0.534 g/cm3 |
| Melting point | 180.5 ℃ |
| Boiling point | 1,342 ℃ |
| Main isotopes | 6Li, 7Li |
| Natural occurrence | Primordial; found in minerals like spodumene and in brines |
| Discovered by | Johan August Arfwedson, 1817 |
History

In the 1790s, the first lithium mineral, petalite LiAlSi4O10, was discovered on the Swedish island of Utö by Brazilian scientist José Bonifácio de Andrada e Silva. Then, in 1817, Johan August Arfvedson of Stockholm examined it and made a groundbreaking discovery: it contained lithium, a metal that was previously unknown. Arfwedson continued his research and later found that this same element was also present in the minerals spodumene and lepidolite.
Arfwedson and Christian Gmelin both made attempts to extract pure lithium from its salts, with Gmelin first observing in 1818 that flames produced by lithium salts have a vivid red color. However, their efforts were unsuccessful. It wasn’t until 1821 that William Thomas Brande succeeded in isolating the element by electrolyzing lithium oxide. This technique was already known to chemist Sir Humphry Davy, who had used it to separate other alkali metals such as sodium and potassium.
Robert Bunsen and Augustus Matthiessen made a significant breakthrough in 1855 by electrolyzing lithium chloride to produce more lithium. This discovery led to the German company Metallgesellschaft AG producing lithium on a large scale starting in 1923, using a liquid solution of lithium chloride and potassium chloride. Fast forward to 1949, when Australian psychiatrist John Cade is credited with reintroducing and popularizing the use of lithium to treat mania. Its potential to stabilize mood and treat both mania and depression quickly gained popularity in Europe and the US in the mid-20th century.
Occurrence
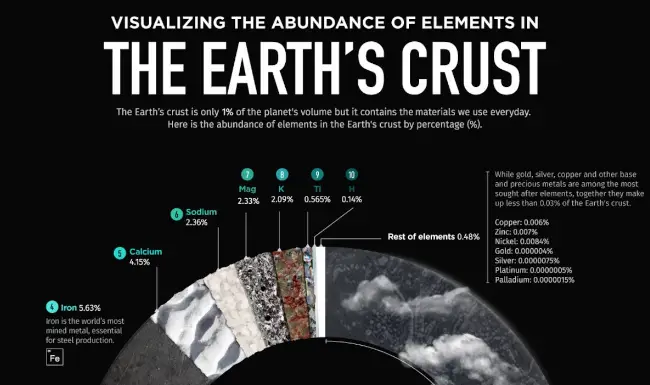
According to modern cosmological theory, lithium is believed to have been one of three elements synthesized during the Big Bang, along with beryllium and boron. Lithium is found in brown dwarf substellar objects and some irregular orange stars as well. Despite its widespread distribution throughout the planet, lithium does not exist in an elemental state naturally due to its high reactivity. Lithium is present in the Earth’s crust at a concentration of roughly 20 milligrams per kilogram, accounting for only 0.002% of the Earth’s crust. This places it as the 25th most abundant element on the planet.
While lithium is a relatively rare element, it can be found in many types of rocks and some brines. However, its abundance in these sources is often limited and only present in very small quantities. Though there are abundant lithium mineral and brine resources, only a small percentage of them are commercially viable. Many of these resources are either too small or of insufficient quality to be of practical use.[1]
Lithium can be found in trace amounts in a wide variety of organisms, including plants, plankton, and invertebrates, with concentrations ranging from 69 to 5,760 parts per billion (ppb). While vertebrates have slightly lower concentrations of lithium, it is still present in almost all bodily fluids and tissues, typically ranging from 21 to 763 ppb.
Production
Lithium metal is typically produced through the process of electrolyzing a mixture of fused lithium chloride and potassium chloride at a temperature of around 450 ℃. However, the majority of the world’s lithium supply is obtained from brines and ores. As of 2018, several countries are major producers of lithium, with the top five being Australia, Chile, China, Argentina, and Zimbabwe.
In the United States, lithium is primarily extracted from brine pools in Nevada. However, as of 2019, the top four lithium-producing countries in the world are Australia, Chile, China, and Argentina, according to the US Geological Survey. The Lithium Triangle, which includes the countries of Chile, Bolivia, and Argentina, is a region known for its significant lithium reserves. In fact, it’s estimated that over 75% of the world’s known lithium reserves are located in the Lithium Triangle.
In July 2018, a high-grade lithium deposit of 2.5 million tonnes and 124 million pounds of uranium resources were discovered in the Falchani hard rock deposit in the Puno region.[2] Historically, lithium and its compounds were primarily isolated and mined from hard rock deposits. However, by the 1990s, mineral springs, brine pools, and brine deposits had become the dominant sources for lithium production.
Properties
Physical properties
Density, melting point, and boiling point
Lithium, among the alkali metals, has the lowest density of 0.534 g/cm3, as well as the highest melting point at 180.50 ℃ and the highest boiling point at 1330 ℃.
Phase change transformation
Similar to sodium, lithium experiences diffusionless phase change transformations below 70 K.
Chemical properties
Reactivity
Lithium is much more reactive when it is molten than when it is solid. Although lithium is the least reactive of the alkali metals, it is still a highly reactive element.
Oxidation

When freshly cut, lithium has a shiny, silvery-white appearance, but it quickly tarnishes to a dull gray color as it reacts with oxygen to form a layer of lithium oxide on its surface.
Conductivity
Lithium is a good conductor of heat and electricity.
Crystal system
Lithium possesses a rhombohedral crystal system at 4.2 K, which changes to a face-centered cubic and then a body-centered cubic at higher temperatures.
Other properties
Floatation
Along with sodium and potassium, lithium is one of only three metals that can float on water.
Applications
Industrial
Grease made with lithium soap is a popular and versatile lubricant, known for its ability to thicken oils and perform well at high temperatures.
Lithium’s high specific heat capacity value of 3.58 kilojoules per kilogram-kelvin makes it a preferred coolant for heat transfer applications.[3][4]
Foundries often use lithium compounds as additives (fluxes) to foundry sand for iron casting to reduce veining and improve the quality of the castings.
Metallic lithium is commonly used as a flux for welding and soldering, due to its ability to promote the fusing of metals and prevent the formation of oxides by absorbing impurities.
Lithium chloride and lithium bromide are frequently utilized as desiccants in gas streams, thanks to their hygroscopic properties that make them effective at removing moisture from the air.
Military and aerospace
In thermonuclear weapons, lithium-6 containing lithium hydride is utilized as a fuel for the fusion stage of the bomb.
Lithium hydroxide and lithium peroxide are commonly used as air purifiers in confined spaces such as submarines and spacecraft to remove carbon dioxide and maintain the quality of the air.
Pyrotechnics
Lithium compounds are often used as pyrotechnic colorants and oxidizers in various applications such as red fireworks and flares. When ignited, these compounds emit a vibrant red hue, making them popular in pyrotechnic shows.
Electronics

Lithium is primarily used in the production of lithium-ion batteries, which are used in mobile phones and electric vehicles. There are various types of lithium-based rechargeable batteries, including lithium-ion polymer, lithium iron phosphate, and nanowire batteries. Lithium-ion batteries have become the industry standard for portable electronics, with around 90% of laptops and 60% of cell phones using them.
In addition to its use in batteries, lithium has been found to aid in the production of silicon nano-welds in electronic components for batteries and other devices.
Medical
Lithium is an effective treatment for bipolar disorder, a mental illness characterized by manic and depressive episodes. Studies have shown that lithium therapy has no significant impact on a patient’s body weight, making it a preferred treatment option for those concerned about weight gain.[5]
Organic synthesis
Lithium compounds are widely used as reagents in the preparation of organic compounds. Some common examples include lithium triethylborohydride, lithium aluminum hydride, n-butyllithium, and tert-butyllithium.
Optical
Lithium fluoride crystals, produced artificially, are transparent and clear, and are commonly used in optics for infrared (IR) and ultraviolet (UV) applications due to their excellent transmittance properties.
Interesting facts
Lithium has the lowest density of all metals, with a density that is approximately half that of water, causing it to float on water.
Lithium is a soft metal that can be easily cut with a knife.
When lithium comes into contact with air, it burns with a red-colored flame.
Lithium is not found in nature in its free form and is only found in igneous rocks.
Due to its vigorous reaction with air and water, lithium is typically stored in oil to prevent its reaction with water.
Related
More elements
References
- The Trouble with Lithium 2 – Lomiko Metals
- Plateau Energy Metals Peru unit finds large lithium resources – Reuters
- SPECIFIC HEAT OF SOLIDS – Internet Archive
- LITHIUM LITERATURE REVIEW: LITHIUM’S PROPERTIES AND INTERACTIONS – OSTI.GOV (.gov)
- Lithium therapy and weight change in people with bipolar disorder: A systematic review and meta-analysis – ScienceDirect
External links
- Lithium – Wikipedia
- Lithium – Element information, properties and uses – Royal Society of Chemistry
- Lithium | Definition, Properties, Use, & Facts – Britannica
- Lithium – Minerals Education Coalition
- What Is Lithium? – Live Science
- Lithium | Li (Element) – National Institutes of Health (.gov)
- Lithium – 3 Li: the essentials – WebElements
- Lithium – an overview – ScienceDirect
- Lithium Definition & Meaning – Merriam-Webster
- Lithium (Li) – Chemical properties, Health and Environmental effects – Lenntech
- Chemistry of Lithium (Z=3) – Chemistry LibreTexts
- It’s Elemental – The Element Lithium – Science Education at Jefferson Lab
- Lithium Element Facts – Chemicool
- Lithium Facts: Li or Element 3 – ThoughtCo
- #3 – Lithium – Li – School City of Hobart
- Lithium Facts – Science Notes and Projects
- Lithium: Uses, Properties and Interesting Facts – Chemistry Dictionary
- Chemistry for Kids: Elements – Lithium – Ducksters
- Lithium | Li Neutrons, Protons & Mining – Study.com
- Lithium: General Info and Everyday Items – Chem4Kids
- Lithium (Li) – American Elements
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
