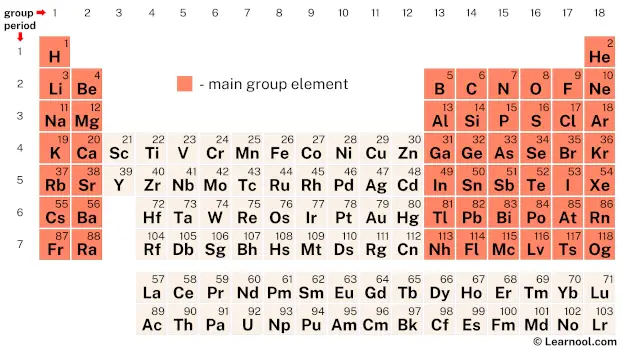
Main-group elements, also known as representative elements, encompass a significant portion of the periodic table. They include elements from group 1 and 2, which form the s-block, as well as elements from group 13 to 18, which form the p-block. It’s worth noting that hydrogen is sometimes classified separately from the main-group elements due to its unique properties and behavior. These elements exhibit various characteristics, including the ability to have multiple oxidation states. For example, oxygen (O) can have oxidation states of -2 or +2, while chlorine (Cl) can have oxidation states of -1, +1, +3, +5, or +7.
The main-group elements include some of the lightest members of the periodic table, such as helium (He), lithium (Li), beryllium (Be), boron (B), carbon (C), nitrogen (N), oxygen (O), and fluorine (F). Each of these elements possesses unique properties and finds applications in diverse fields. For example, helium is used in cryogenics and as a lifting gas, lithium is utilized in rechargeable batteries, and carbon is the fundamental element in organic chemistry and forms the basis of all known life forms. The main-group elements play vital roles in numerous natural processes and are integral to the functioning of various industries and technologies.
Main-group elements, along with the lighter transition metals, are abundantly found in the universe, the solar system, and the Earth’s crust. Their presence in significant quantities has earned them the name “representative elements.” For instance, silicon (Si) and aluminum (Al) are two main-group elements that make up a significant portion of its composition in the Earth’s crust. Their abundance contributes to their importance in various natural processes and their widespread use in a variety of applications.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – main-group element |
On the periodic table, main-group elements encompass a wide range of chemical elements. This includes alkali metals, alkaline earth metals, and others located in the p-block, specifically from group 13 to 18. Hydrogen, although sometimes excluded, is also considered a main-group element.
Related
More topics
- Transuranium element
- Superheavy element
- Rare-earth element
- Synthetic element
- Main-group element
- Inner transition metal
External links
- Main-group element – Wikipedia
- Main Group Elements – ChemTalk
- Main Group Elements – Definition and Importance – Science Notes and Projects
- 8.2: What are the main group elements and why should anyone care about them? – Chemistry LibreTexts
- 14 – PERIODIC TRENDS IN THE MAIN GROUP ELEMENTS – Berkeley City College
- Main Group Elements: Definition, List, and Importance – Chemistry Learner
- The Main Group Elements – Angelo State University
- Main Group Elements Definition – ThoughtCo
- Periodic table, main group elements – New World Encyclopedia
- the Main-Group Metals – University Science Books
- Periodic Table – An Introduction to Chemistry
- Representative Elements | Definition, List & Groups – Study.com
- Main Group Element – an overview – ScienceDirect
- 7.2 The Periodic Table – Human Biology – University of Minnesota
- Main-group elements as transition metals – Nature Journal
- Where in the periodic table are the main-group elements found? Where are the transition metal groups found? – Pearson
- Elements – Florida State College
- 19 Fascinating Facts About Main Group Element – Facts.net
- Transition Metals – Purdue University
- Nitrogen fixation and transformation with main group elements – RSC Publishing
- What are the main group elements of the periodic table? – Answers
- Main Group Element – Academic Accelerator
- Electron Configurations of Main Group Elements – CK-12 Foundation
- Main Group Elements Flashcards – Quizlet
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
