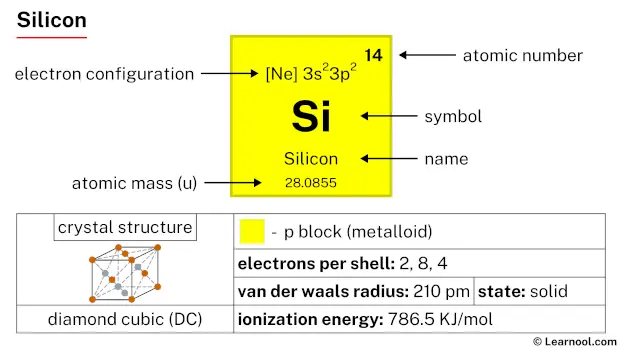
Silicon (Si) is a chemical element of the periodic table, located in the group 14 and the period 3, and is having the atomic number 14. It is a hard, brittle, lustrous, dark-grey metalloid, whose name comes from the Latin word “silex” or “silicis”, which means flint or hard stone. It is the second most abundant element on earth. It is a member of the carbon group.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – p block |
Silicon is a p-block element, found in the fourteenth column (carbon group) and the third row of the periodic table. It has the atomic number 14 and is denoted by the symbol Si.
Element information
| Origin of name | Latin word “silex” or “silicis” (which means flint or hard stone) |
| Symbol | Si |
| Atomic number (Z) | 14 |
| Atomic mass | 28.0855 u |
| Block | p-block |
| Group | 14 (carbon group) |
| Period | 3 |
| Classification | Metalloid |
| Atomic radius | 111 pm |
| Covalent radius | 111 pm |
| Van der Waals radius | 210 pm |
| Melting point | 1414 ℃, 2577 ℉, 1687 K |
| Boiling point | 3265 ℃, 5909 ℉, 3528 K |
| Electron configuration | [Ne] 3s2 3p2 |
| Learn how to write: Silicon electron configuration | |
| Electrons per shell | 2, 8, 4 |
| Learn how to draw: Silicon Bohr model | |
| Crystal structure | Face-centered diamond-cubic |
| Phase at r.t | Solid |
| Density near r.t | 2.3290 g/cm3 |
| Main isotopes | Silicon-28, Silicon-29, Silicon-30 |
| Natural occurrence | Primordial |
| Oxidation state | -4, +4 |
| Electronegativity (Pauling scale) | 1.90 |
| Protons Neutrons Electrons |
14 14 14 |
| Learn how to find: Silicon protons neutrons electrons | |
| Valence electrons | 4 |
| Learn how to find: Silicon valence electrons | |
| CAS number | 7440-21-3 |
| Discovered by | Jöns Jacob Berzelius in 1824 |
History

The discovery and naming of silicon are credited to several chemists, including Antoine Lavoisier, Sir Humphry Davy, and Jöns Jacob Berzelius. Lavoisier first suspected that silica might be an oxide of a fundamental chemical element in 1787, but it was not until 1817 that Thomas Thomson gave it the name “silicon,” adding the suffix “-on” to indicate that it was a nonmetal. Berzelius is typically credited with the discovery of the element because he purified and characterized it as a new element in 1824.
Silicon in its crystalline form was not isolated until 1854, when Henri Etienne Sainte-Claire Deville electrolyzed a mixture of sodium chloride and aluminum chloride containing approximately 10% silicon, producing a slightly impure allotrope of silicon. More cost-effective methods have since been developed to isolate several allotropic forms, including the most recent discovery of silicene in 2010.
In the early 20th century, the chemistry and industrial use of siloxanes and silicone polymers, elastomers, and resins were developed, leading to the widespread use of silicon in a range of products, from sealants and adhesives to medical implants and electronics. The solid-state physics of doped semiconductors and the crystal chemistry of silicides were also mapped in the late 20th century, paving the way for the development of modern electronic devices such as computers and smartphones. Today, silicon remains one of the most important elements in the world, with a wide range of applications across multiple industries.
Occurrence
Silicon is the second most abundant element in the Earth’s crust, after oxygen, and is found mainly in the form of silicon dioxide (SiO2), which is commonly known as silica. Silicon also occurs in various other minerals, such as feldspars, micas, and clays. It is also found in some types of rocks, such as granite, gneiss, and sandstone.
Silicon can also be found in living organisms, especially in plants and animals. It is an essential element for many organisms, including humans. Silicon plays a role in the formation of bones, teeth, and connective tissues, and is also important for the growth and development of plants.
Production
Silicon is typically produced by reducing silica (SiO2) with carbon in a furnace. The process involves heating a mixture of silica, carbon, and a reducing agent, such as wood chips or coal, in an electric arc furnace at temperatures of around 2000 ℃. The silicon produced is then typically purified by several refining steps, including acid leaching, filtration, and distillation.
Another common method of producing silicon is through the thermal decomposition of silane (SiH4) gas. Silane is first produced by reacting metallurgical grade silicon with hydrogen at high temperatures. The resulting silane gas is then decomposed at high temperatures to produce pure silicon.
There are also several other methods of producing silicon, including the use of the chemical vapor deposition (CVD) process and the fluidized bed reactor process. The CVD process involves the reaction of a silicon-containing gas, such as silane or silicon tetrachloride, with a substrate surface at high temperatures, while the fluidized bed reactor process involves the reduction of silicon dioxide with a reducing gas, such as hydrogen, in a fluidized bed reactor.
Properties
Physical properties
Silicon is a hard, brittle crystalline solid with a blue-gray metallic luster.
It has a high melting and boiling point.
It is a poor conductor of electricity and heat.
Chemical properties
Silicon is a semiconductor with an electrical conductivity between that of a conductor and an insulator.
It reacts with halogens (fluorine, chlorine, bromine, iodine) to form silicon tetrahalides.
It does not react with water, but reacts with steam to form silicon dioxide and hydrogen gas.
It is not affected by acids except for hydrofluoric acid.
Crystal structure
Silicon has a diamond cubic crystal structure.
Each silicon atom is tetrahedrally coordinated with four neighboring silicon atoms.
Isotopes
Silicon has three stable isotopes: 28Si, 29Si, and 30Si.
28Si is the most common isotope of silicon, making up about 92.2% of the natural abundance.
Allotropes
Silicon has several allotropes, including amorphous, crystalline, and black (or metallic) silicon.
Optical properties
Silicon is transparent to infrared radiation.
It has a high refractive index and can be used in lenses and prisms.
Mechanical properties
Silicon is a hard and brittle material.
It has a high Young’s modulus and is used in the construction of microelectromechanical systems (MEMS).
Applications
Semiconductors
Silicon is the most widely used material in the production of semiconductors. Its unique electronic properties, including its ability to conduct electricity under certain conditions, make it a crucial component in the manufacture of integrated circuits, transistors, and other electronic devices.
Solar cells
Silicon is also widely used in the production of solar cells. It is the primary material used in the manufacture of photovoltaic cells, which convert sunlight into electricity.
Glass and ceramics
Silicon dioxide (SiO2), also known as silica, is a key component in the production of glass and ceramics. Silicon is also used to make synthetic quartz crystals, which are used in watches and other electronic devices.
Construction
Silicon-based materials are used in a wide range of construction applications. For example, silicon is used to make high-strength, lightweight concrete, and is also used in the production of sealants and adhesives.
Medical implants
Silicon is biocompatible, meaning it does not cause an adverse reaction when implanted in the human body. This property makes it an ideal material for medical implants such as pacemakers, artificial joints, and other medical devices.
Lubricants
Silicon-based lubricants are used in a variety of applications, including automotive, aerospace, and industrial machinery. These lubricants provide superior performance under extreme conditions and are often used in high-temperature or high-pressure environments.
Cosmetics
Silicones, which are derived from silicon, are used in a wide range of cosmetic products. They are often used as emollients, which help to moisturize and soften the skin, and as thickeners, which give cosmetic products their smooth, creamy texture.
Interesting facts
Silicon is the second most abundant element in the Earth’s crust, making up about 28% of its mass.
Silicon has a high melting point of 1414 ℃ and a boiling point of 3265 ℃.
Silicon is a semiconductor, which means it can conduct electricity under certain conditions and is used extensively in electronic devices.
Silicon has a diamond-like crystal structure and is brittle in nature.
Silicon is used in the production of glass, ceramics, and cement.
Silicon has a unique ability to form silicon-oxygen bonds, which makes it a key element in the formation of silicates, the most common minerals on Earth.
Silicon has isotopes that are used in various medical applications, including diagnosing and treating cancer.
Silicon is also used in the production of solar cells, as it can convert light into electricity.
The element silicon was first isolated in 1824 by Jöns Jacob Berzelius, a Swedish chemist.
The name “silicon” comes from the Latin word “silicis,” which means “flint” or “hard stone.”
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/14/silicon
- https://www.britannica.com/science/silicon
- https://en.wikipedia.org/wiki/Silicon
- https://education.jlab.org/itselemental/ele014.html
- https://pubchem.ncbi.nlm.nih.gov/element/Silicon
- https://www.chemicool.com/elements/silicon.html
- https://chemistrytalk.org/silicon-element/
- https://www.livescience.com/28893-silicon.html
- https://www.radiochemistry.org/periodictable/elements/14.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.