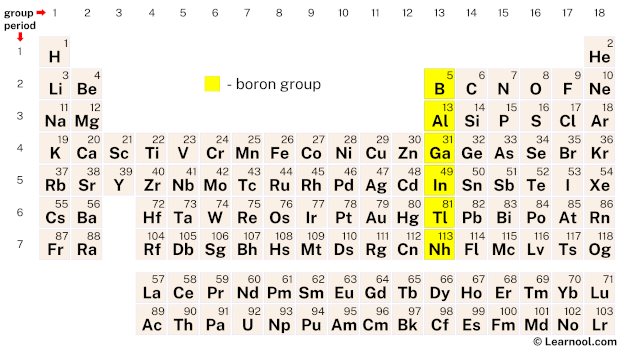
The boron group, also known as the triels, comprises six elements in the periodic table: boron (B), aluminum (Al), gallium (Ga), indium (In), thallium (Tl), and nihonium (Nh). These elements are characterized by having three valence electrons. Boron, being the lightest element in the boron group, is a metalloid, while the other elements (excluding nihonium) are classified as post-transition metals.
These elements are located in the p-block of the periodic table. In terms of abundance rank, aluminum is the most abundant element in the group, constituting approximately 8.1% of the Earth’s crust. Boron, although relatively rare, occurs at about 10 ppm (parts per million) and is known for its unique properties. Gallium, with an abundance of about 19 ppm, and indium, with an abundance of about 0.1 ppm, are relatively scarce compared to aluminum. Thallium is even rarer, occurring at approximately 0.7 ppm. It’s worth noting that nihonium is a synthetic element, meaning it does not occur naturally and is produced through artificial processes.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – boron group |
On the periodic table, the boron group – consisting of six chemical elements: boron, aluminum, gallium, indium, thallium, and nihonium – is located in group 13.
Related
More topics
- Boron group
- Carbon group
- Nitrogen group
- Oxygen group
- Halogen
- Noble gas
External links
- Boron group – Wikipedia
- Boron group element | Properties & Facts – Britannica
- Group 13: The Boron Family – Chemistry LibreTexts
- boron group – Wiktionary
- About: Boron group – DBpedia
- Boron group – wikidoc
- The Boron Group | The Aqueous Chemistry of the Elements – Oxford Academic
- Boron group Facts for Kids – Kids encyclopedia facts
- Group 13 Elements: The Boron Family – Pearson
- The Boron Group – Group 13 – Wiley Online Library
- Boron Family – Carbon Family – Nitrogen Family – Concept – Brightstorm
- Boron Group – Academic Accelerator
- Group 13 Elements – Bartleby
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.





