
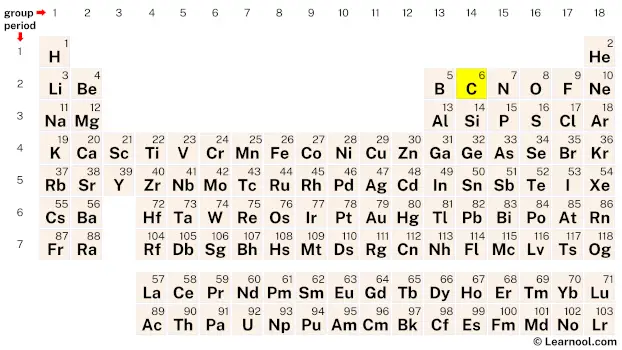
Carbon (C) is a chemical element of the periodic table, located in the group 14 and the period 2, and is having the atomic number 6. It is a shiny, black or dull grey reactive nonmetal, whose name comes from the Latin word “carbo”, which means charcoal. It is the 17th most abundant element on earth and is the first element in the carbon group.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – p block |
Carbon is a p-block element, situated in the fourteenth column and the second row of the periodic table, denoted by the atomic number 6 and chemical symbol C.
Element information
 |
|
 |
|
| Origin of name | Latin word “carbo” (which means charcoal) |
| Symbol | C |
| Atomic number (Z) | 6 |
| Atomic mass | 12.0107 u |
| Block | p-block |
| Group | 14 (carbon group) |
| Period | 2 |
| Classification | Reactive nonmetal |
| Covalent radius | sp3: 77 pm sp2: 73 pm sp: 69 pm |
| Van der Waals radius | 170 pm |
| Sublimation point | 3642 ℃, 6588 ℉, 3915 K |
| Electron configuration | [He] 2s2 2p2 |
| Electrons per shell | 2, 4 |
| Learn how to draw: Carbon Bohr model | |
| Crystal structure | Graphite: simple hexagonal (black) Diamond: face-centered diamond-cubic (clear) |
| Phase at r.t | Solid |
| Density near r.t | Amorphous: 1.8-2.1 g/cm3 Graphite: 2.267 g/cm3 Diamond: 3.515 g/cm3 |
| Main isotopes | Carbon-12, Carbon-13 |
| Natural occurrence | Primordial |
| Oxidation state | -4, +1, +2, +3, +4 |
| Electronegativity (Pauling scale) | 2.55 |
| Protons Neutrons Electrons |
6 6 6 |
| Learn how to find: Carbon protons neutrons electrons | |
| Valence electrons | 4 |
| Learn how to find: Carbon valence electrons | |
| CAS number | Atomic carbon: 7440-44-0 Graphite: 7782-42-5 Diamond: 7782-40-3 |
| Discovered by | Egyptians and Sumerians in 3750 BCE |
History
Carbon is a non-metallic element that has been known since ancient times. The word “carbon” comes from the Latin word “carbo,” meaning charcoal.
The discovery of carbon is attributed to several ancient civilizations. The Egyptians and the Sumerians were known to use carbon in the form of charcoal for smelting metals. The Chinese, on the other hand, used carbon black as a pigment for writing and drawing.
In the 18th century, the French chemist Antoine Lavoisier conducted experiments on carbon and discovered that it was an element. He showed that carbon dioxide was produced when carbon was burned in air, and he named this gas “fixed air.” In the early 19th century, the British chemist Sir Humphry Davy discovered that diamond, a form of carbon, could be electrically conductive.
The study of carbon continued to develop throughout the 19th and 20th centuries, leading to the discovery of numerous carbon-based compounds and the development of organic chemistry. Today, carbon is a fundamental element in many fields, including materials science, biology, and energy research.
Occurrence and production
Carbon is the fourth most abundant element in the universe and the fifteenth most abundant element in the Earth’s crust. It is widely distributed throughout nature and occurs in various forms, including diamond, graphite, coal, charcoal, and carbon dioxide. Carbon also occurs in all living organisms, where it is an essential component of biomolecules such as proteins, carbohydrates, and nucleic acids.
Carbon is produced by a variety of methods, including the decomposition of organic matter, the combustion of fossil fuels, and the heating of carbon-containing materials such as wood or coal in the absence of oxygen (pyrolysis). It can also be produced through industrial processes such as the reduction of carbon dioxide, the carbothermic reduction of metal oxides, and the cracking of hydrocarbons. In addition, carbon can be synthesized through a process known as chemical vapor deposition, in which carbon-containing gases are passed over a substrate to deposit a layer of carbon. The production of high-purity carbon materials such as diamond and graphite involves specialized methods such as high-pressure high-temperature synthesis and graphite intercalation.
Properties
Physical properties
Carbon exists in several allotropes, including diamond, graphite, and fullerenes.
Diamond is the hardest known naturally occurring substance, has a high melting point, and is transparent to visible and ultraviolet light.
Graphite is a soft, black, slippery solid that is used in pencils and as a lubricant.
Carbon has a sublimation point of 3642 ℃, which means it transitions directly from a solid to a gas state without passing through a liquid phase.
It has a density of 2.267 g/cm3.
Chemical properties
Carbon readily reacts with oxygen to form carbon dioxide and carbon monoxide.
It forms covalent bonds with other atoms, including carbon atoms to form long chains and rings in organic molecules.
Carbon is capable of forming double and triple bonds with other atoms, making it a versatile element in organic chemistry.
Carbon can also form coordination complexes, in which it donates electrons to transition metals.
Carbon has a strong affinity for hydrogen, and the study of carbon-hydrogen bonds is an important aspect of organic chemistry.
Other properties
Carbon has a relatively low atomic weight of 12.01 and is the fourth most abundant element in the universe.
It is essential for life, as all known living organisms are based on carbon-based molecules.
Carbon is a good electrical conductor in its graphite form, and is used in electrodes for batteries and fuel cells.
Applications
Carbon is used as a fuel in the form of coal, charcoal, and coke. These fuels are used to generate electricity and heat in industries.
Carbon is an essential component in the production of steel. It is used to remove impurities from iron ore in the blast furnace process and to control the carbon content of the steel during production.
Carbon fiber is a strong and lightweight material made from carbon. It is used in aerospace, automotive, and sports equipment industries to make lightweight and strong structures.
Graphite, a form of carbon, is used as a lubricant in machines and as a heat-resistant material in the nuclear industry.
Activated carbon is a highly porous form of carbon that is used to remove impurities from water and air. It is also used in the purification of drugs and as an adsorbent in gas masks.
Diamonds are a form of carbon that are used in jewelry and cutting tools.
Carbon is used in electronics as a material for resistors and capacitors.
Carbon is an essential element for the growth of plants and is used in fertilizers.
Carbon-based materials are used in medical implants and drug delivery systems.
Carbon nanotubes and graphene, both made from carbon, have unique properties that make them useful in various applications, including electronics and energy storage.
Interesting facts
Carbon is the fourth most abundant element in the universe, after hydrogen, helium, and oxygen.
The name “carbon” comes from the Latin word “carbo,” which means “coal.”
Carbon is the basis for all known life on Earth. It forms the backbone of organic molecules such as proteins, DNA, and carbohydrates.
Carbon is unique in its ability to form long chains of itself and with other elements. This allows for the creation of an enormous variety of organic compounds, including synthetic materials such as plastics and rubber.
Diamond, one of the forms of carbon, is the hardest natural substance known to man and has the highest thermal conductivity of any material.
Carbon can also form other interesting allotropes, such as graphite, graphene, and fullerenes.
Carbon has been used in various forms throughout history, from early uses such as writing and drawing with charcoal, to modern applications such as fuel cells and nanotechnology.
Carbon dating, a process used to determine the age of archaeological artifacts, works by measuring the amount of radioactive carbon-14 in the sample.
Carbon is crucial for mitigating climate change, as it plays a major role in the carbon cycle and carbon sequestration.
Related
More elements
External links
- https://en.wikipedia.org/wiki/Carbon
- https://www.rsc.org/periodic-table/element/6/carbon
- https://www.britannica.com/science/carbon-chemical-element
- https://pubchem.ncbi.nlm.nih.gov/element/Carbon
- https://www.livescience.com/28698-facts-about-carbon.html
- https://www.chemicool.com/elements/carbon.html
- https://education.jlab.org/itselemental/ele006.html
- https://chemistrytalk.org/carbon-element/
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
