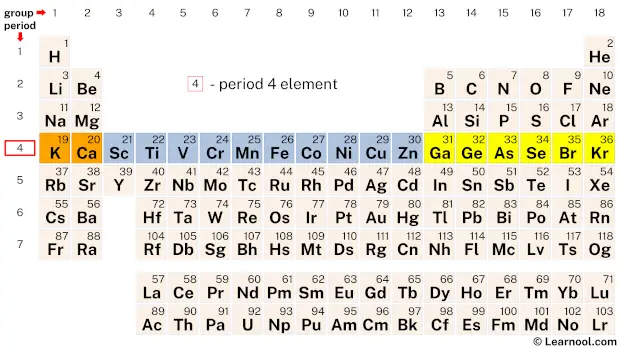
Period 4 elements refer to the chemical elements found in the fourth row or period of the periodic table. This period includes elements ranging from potassium (K) to krypton (Kr), with atomic numbers 19 to 36. Period 4 elements are interesting because they show a range of physical and chemical properties that are distinct from other periods. In particular, these elements have unique electronic configurations that give them different reactivity patterns and physical characteristics.
Period 4 elements display a trend wherein, as we progress across the period, the atomic size decreases, while both electronegativity and ionization energy increase. This means that the elements on the left side of the period, such as potassium and calcium, are highly reactive and tend to lose electrons to form cations, while elements on the right side, such as bromine and krypton, are less reactive and tend to gain electrons to form anions. Period 4 elements also exhibit a variety of metallic and non-metallic properties. For example, metals such as iron and copper are highly conductive, while nonmetals such as selenium, bromine, and krypton are utilized in various chemical applications.
Period 4 elements in the p-block typically adhere to the octet rule, but gallium is an interesting exception; it can have fewer than 8 electrons in its valence shell. Elements such as potassium, calcium, and transition metals in period 4 do not conform to the octet rule, while elements starting from arsenic and beyond can have an expanded octet, meaning they can hold more than eight electrons in their valence shell.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| 4 | – period 4 element |
Period 4 is the fourth row of elements on the periodic table, consisting of 18 elements, including the transition metals such as iron, copper, and nickel, as well as nonmetals such as selenium and bromine.
| Potassium (K) | |
| Calcium (Ca) | |
| Scandium (Sc) | |
| Titanium (Ti) | |
| Vanadium (Va) | |
| Chromium (Cr) | |
| Manganese (Mg) | |
| Iron (Fe) | |
| Cobalt (Co) | |
| Nickel (Ni) | |
| Copper (Cu) | |
| Zinc (Zn) | |
| Gallium (Ga) | |
| Germanium (Ge) | |
| Arsenic (As) | |
| Selenium (Se) | |
| Bromine (Br) | |
| Krypton (Kr) | |
Element information
s-block
Potassium

Potassium is a soft, silvery-white metal that belongs to the alkali metal group of elements. Potassium is highly reactive and is rarely found in its pure elemental form in nature, but is commonly found in various minerals. Potassium is an essential element for all living organisms and is involved in various physiological processes, such as muscle contraction and nerve function. It is also used in the production of fertilizers, soaps, and other chemicals.
Calcium

Calcium is an alkaline earth metal that is silvery-white in appearance. Calcium is essential for many biological processes, including bone formation and muscle contraction. It is also used in the production of steel, aluminum, and other metals. Calcium is commonly found in various minerals, such as limestone and gypsum, and is also present in many foods, such as milk and cheese.
d-block
Scandium

Scandium is a silvery-white metal that has an atomic number of 21 and is the first element in the transition metal series. Scandium is a relatively rare element and is not found in large quantities in the Earth’s crust. It is mainly obtained from minerals such as thortveitite, euxenite, and gadolinite. Scandium has a low density and a high melting point, making it a useful material in aerospace and defense applications. It is also used in the production of high-intensity lamps and as an additive to aluminum alloys to improve their strength and corrosion resistance.
Titanium

Titanium is a strong, lightweight, and corrosion-resistant metal that has an atomic number of 22. Titanium is the ninth most abundant element in the Earth’s crust and is found in minerals such as ilmenite, rutile, and titanite. It has a high melting point and is resistant to corrosion by seawater, making it a valuable material in the aerospace, medical, and chemical industries. Titanium is also used in the production of golf clubs, bicycle frames, and other sporting equipment due to its strength and lightness.
Vanadium
Vanadium is a hard, silvery-gray metal that is primarily used as an alloying element in the production of steel. Vanadium has a high melting point and is relatively rare in the Earth’s crust. It forms a range of oxidation states, with the +2, +3, +4 and +5 states being the most common. Vanadium is also used in the production of ceramics, glass and chemicals.
Chromium

Chromium is a hard, lustrous, steel-gray metal that is highly resistant to corrosion. Chromium is commonly used in the production of stainless steel, which is used in a wide range of applications due to its resistance to corrosion and staining. It is also used in the production of alloys, such as chromium-molybdenum, which are used in the aerospace and automobile industries.
Manganese

Manganese is a gray-white metal that is hard and brittle. Manganese is used primarily as an alloying element in the production of steel, where it improves the strength, hardness, and durability of the steel. It is also used in the production of batteries, ceramics, glass, and fertilizers. Manganese has a wide range of oxidation states, with the +2, +4, and +7 states being the most common.
Iron

Iron is a transition metal that is the fourth most abundant element in the Earth’s crust. Iron has a silvery-gray appearance and is a relatively soft metal that is both ductile and malleable. It has a melting point of 1538 ℃ and a boiling point of 2862 ℃. Iron is known for its strength, durability, and magnetic properties, and it is used in a wide range of applications, including construction, transportation, and manufacturing.
Cobalt

Cobalt is a transition metal that is found in the Earth’s crust and is a hard, lustrous, silver-gray metal. Cobalt has a melting point of 1495 ℃ and a boiling point of 2927 ℃. It is known for its magnetic properties, and it is used in a variety of applications, including in the production of rechargeable batteries, magnets, and high-strength alloys. Cobalt is also an essential element in the human diet, as it is a component of vitamin B12.
Nickel

Nickel is a transition metal that is found in the Earth’s crust and is a hard, lustrous, silvery-white metal. Nickel has a melting point of 1455 ℃ and a boiling point of 2730 ℃. It is known for its resistance to corrosion and its ability to withstand high temperatures. Nickel is used in a wide range of applications, including in the production of stainless steel, magnets, and batteries. It is also an essential element in the human diet, as it is a component of various enzymes.
Copper

Copper (Cu) is a transition metal that is soft, malleable, and ductile. It has a reddish-orange color and is a good conductor of electricity and heat. Copper is commonly used in electrical wiring, plumbing, and in the production of coins and decorative items. It is also an essential nutrient for humans and animals and plays a vital role in the formation of red blood cells and the maintenance of the immune system.
Zinc

Zinc (Zn) is a bluish-white, lustrous metal that is brittle at room temperature. It is a moderately reactive metal that can form a protective layer of oxide on its surface, which prevents further corrosion. Zinc is used in a variety of applications, including galvanizing iron and steel to prevent rusting, as a component in brass and other alloys, and in the production of batteries and electronic components. It is also an essential nutrient for humans and animals and plays a role in the immune system and the metabolism of proteins and carbohydrates.
p-block
Gallium

Gallium is a silvery-white metal that is soft and easily cut with a knife. It has a low melting point of 29.76 ℃, which means that it can melt in the palm of your hand. Gallium is used in a variety of applications, including semiconductors, LEDs, and solar cells. It is also used in the production of alloys, such as aluminum-gallium alloys, which are used in the aerospace industry.
Germanium

Germanium is a grayish-white metalloid that is commonly used in semiconductors and fiber optics. It has a similar crystal structure to diamond and is an excellent semiconductor material. Germanium is also used in infrared optics, as a phosphor in fluorescent lamps, and in the production of some types of alloys.
Arsenic

Arsenic is a gray, metallic-looking element that is commonly used in the production of semiconductors and in the manufacture of pesticides. It is also used in the production of alloys, such as lead-arsenic alloys, which are used in the construction industry. Arsenic has a long history of use in medicine, although it is now recognized as a toxic substance that can cause cancer and other health problems.
Selenium

Selenium is a nonmetal that has several allotropes, with the most stable form being a gray, metallic-looking solid. Selenium is an essential trace element in many organisms and plays a crucial role in human health, including its antioxidant properties. It is also used in the glass industry, in pigments, and as a photoconductor in photocopiers and other electronic devices.
Bromine

Bromine is a nonmetallic element, the only nonmetallic element that is liquid at room temperature and pressure, and is a reddish-brown, volatile liquid that has a pungent odor. Bromine is a highly reactive element that can form compounds with a variety of elements, including metals and nonmetals. It is used in a variety of applications, including in the production of flame retardants, dyes, and pharmaceuticals.
Krypton

Krypton is a noble gas. It is a colorless, odorless, tasteless gas that is present in the Earth’s atmosphere in trace amounts. Krypton is used in a variety of applications, including in lighting and electronics. It is also used in some medical applications, such as in the production of radioactive isotopes for medical imaging.
Physical and chemical properties
Density
The density of period 4 elements generally increases from left to right across the period. This is due to the increase in atomic mass and the decrease in atomic radius, which leads to a more compact arrangement of atoms. For example, titanium has a density of 4.50 g/cm3, while copper has a density of 8.96 g/cm3.
Atomic radius
The atomic radius of period 4 elements generally decreases from left to right across the period. This is due to the increase in nuclear charge, which attracts the electrons more strongly and pulls them closer to the nucleus. For example, the atomic radius of scandium is 162 pm, while the atomic radius of zinc is only 134 pm.
Metallic character
The metallic character of period 4 elements generally decreases from left to right across the period. This is due to the increase in electronegativity and the decrease in atomic radius, which makes it harder for the outermost electrons to be removed from the atom. For example, potassium and calcium are highly reactive metals, while zinc is a less reactive metal.
Electronegativity
The electronegativity of period 4 elements generally increases from left to right across the period. This is due to the increase in nuclear charge, which attracts the electrons more strongly and makes it harder for them to be removed from the atom. For example, potassium has an electronegativity of 0.82, while bromine has an electronegativity of 2.96.
Ionization energy
The ionization energy of period 4 elements generally increases from left to right across the period. This is due to the increase in nuclear charge, which makes it harder for the electrons to be removed from the atom. For example, the first ionization energy of vanadium is 650.9 kJ/mol, while the first ionization energy of krypton is 1350.8 kJ/mol.
Reactivity
The reactivity of period 4 elements varies depending on the specific element. For example, potassium and calcium are highly reactive metals that can easily lose their outermost electrons to form cations, while selenium is a highly reactive nonmetal that can easily gain electrons to form anions. Other elements, such as titanium and zinc, are less reactive and form more stable compounds.
Related
More topics
- Period 1 element
- Period 2 element
- Period 3 element
- Period 4 element
- Period 5 element
- Period 6 element
- Period 7 element
External links
- Period 4 element – Wikipedia
- Lesson Explainer: Uses of Period 4 Metals Chemistry – Nagwa Limited
- Period 4 element Facts for Kids – Kids encyclopedia facts
- Period 4 element – Elements Wiki | Fandom
- About: Period 4 element – DBpedia
- Period 4 Element – Academic Accelerator
- Period 4 element – wikidoc
- Period 4 Subshell Electronic Configuration Tutorial – AUS-e-TUTE
- How to Write the Electron Configuration for Elements in the 4th Period of the Periodic Table – Study.com
- Period 4 element – Wikiwand
- Period 4 element Facts for Kids – KidzSearch Wiki
- period 4 – Wikidata
- Period 4 Elements Flashcards – Quizlet
- Why do the elements of period 4 start a new cycle of properties after the element argon? – Brainly
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
