
Sodium (Na) is a chemical element of the periodic table, located in group 1 and period 3, and has the atomic number 11. It is a soft, silvery-white alkali metal whose name comes from the English word “soda”. Sodium is the sixth most common element on Earth, making about 2.6 percent of the planet’s crust. It can be found in a variety of minerals, like feldspar and sodalite. Since sodium does not naturally occur, it must be made from other substances. There are 20 known isotopes of sodium, but only 23Na is stable.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – s block |
Sodium is an s-block element, situated in the first column and the third row of the periodic table. Its atomic number is 11 and its symbol is Na.
Element information
| Sodium element | |
|---|---|
| Symbol | Na |
| Atomic number (Z) | 11 |
| Standard atomic weight | 22.98976928 |
| CAS number | 7440-23-5 |
| Origin of name | From the English word soda; symbol from Latin natrium |
| Group | 1 |
| Period | 3 |
| Block | s |
| Classification | Alkali metal |
| Electron configuration | [Ne] 3s1 |
| Electrons per shell | 2, 8, 1 |
| Learn how to draw: Sodium Bohr model | |
| Valence electrons | 1 |
| Learn how to find: Sodium valence electrons | |
| Protons, neutrons, electrons | 11 protons, 12 neutrons, 11 electrons (for most common isotope: 23Na) |
| Learn how to find: Sodium protons neutrons electrons | |
| Oxidation state(s) | +1 |
| Electronegativity (Pauling scale) | 0.93 |
| Atomic radius | 186 pm |
| Covalent radius | 166 pm |
| Van der Waals radius | 227 pm |
| Phase at room temperature | Solid |
| Crystal structure | Body-centered cubic (bcc) |
| Density near room temperature | 0.968 g/cm3 |
| Melting point | 97.79 ℃ |
| Boiling point | 883 ℃ |
| Main isotopes | 23Na |
| Natural occurrence | Primordial; found in minerals such as halite (rock salt) |
| Discovered by | Humphry Davy, 1807 |
History

The term sodium is supposed to derive from the Arabic suda, which means headache, because the headache-relieving qualities of sodium carbonate or soda were well known in ancient times. Sir Humphry Davy was the first to create sodium in its elemental form in 1807, using electrolysis of fused sodium hydroxide (NaOH). He made this discovery known at the Bakerian session at the Royal Society of London on November 19, 1807.[1]
Occurrence
Because of its strong reactivity, sodium is never found as a pure element; instead, it can be found in a variety of minerals like cryolite, amphibole, soda niter (sodium nitrate), zeolite, etc.[2] Sodium is the sixth most abundant element on Earth after oxygen, silicon, aluminum, iron, and calcium, with an earth’s crust abundance of 2.6%. Of the alkali group of metals, it is the most abundant.
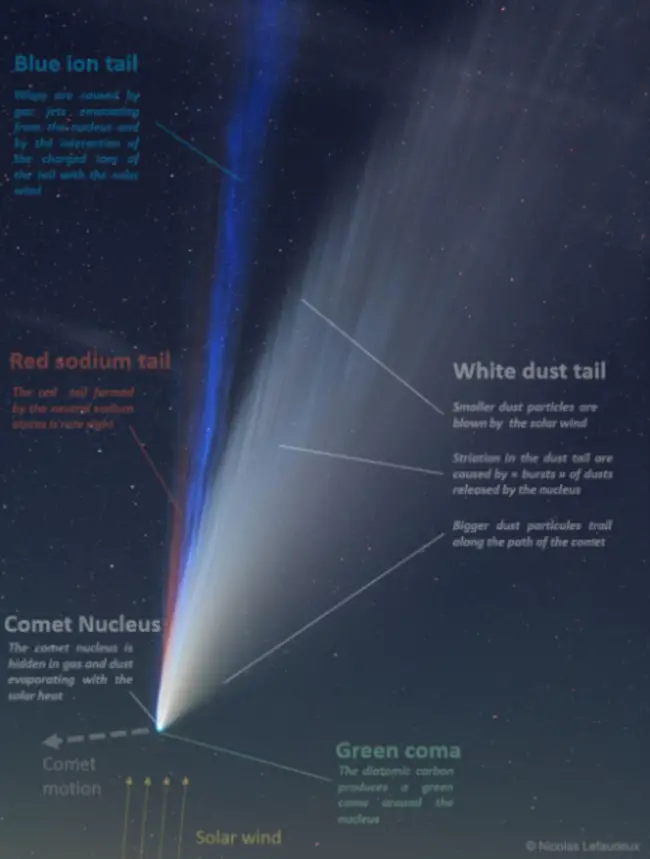
The spectra of stars, including the Sun, and the interstellar medium have both shown the presence of sodium in atomic and ionic form. Not just comets or meteoroids include sodium. Other solar system bodies like Mercury, Io, and the Moon also have sodium.[3] Some comets contain sodium tails, which were originally noticed in observations of Comet Hale-Bopp in 1997. Sodium has even been identified in the atmospheres of certain extrasolar planets.
Production
In the late 19th century, metallic sodium was first commercially manufactured by carbothermal reduction of sodium carbonate at 1100 ℃ as the first stage in the Deville process for the synthesis of aluminum. In 1886, a similar process involving the electrolysis of sodium hydroxide was invented. Today, sodium is produced commercially by electrolyzing completely dry fused sodium chloride. Compared to the earlier Castner process (the electrolysis of sodium hydroxide), this method is more affordable.
Properties

Sodium is a good conductor of heat and electricity, and can easily be cut with a knife.
At standard pressure and temperature, sodium is a soft silvery metal that reacts with air and becomes grayish white sodium oxide.
It never occurs in the free state in the crust of the Earth because it is so reactive. Sodium and its compounds glow yellow when exposed to flame.
Sodium (along with lithium and potassium) is one of only three metals that can float on water. And is the third-least dense of all elemental metals due to its low atomic mass and high atomic radius.
Applications
Sodium serves as a raw ingredient in the production of sodium hydride (NaH) and sodium borohydride (NaBH4).
It is widely employed in metallurgy as a deoxidant and as a reducing agent for the manufacture of calcium, zirconium, titanium, and other transition metals.
Sodium borohydride, sodium azide, indigo, and triphenylphosphine are primarily produced using metallic sodium.
Molten sodium has been used as a coolant in liquid-metal fast breeder reactors because it is a good heat-transfer fluid.
Outdoor places like parking lots, security checkpoints, airports, and other public spaces are lit by sodium vapor, which emits a dazzling yellow light.
Sodium chloride is extensively used for anti-icing and de-icing, added to food to improve flavor, and has also been used as a preservative to prevent food from spoiling.
Sodium is used as a feedstock in chemical industries, as a water softener, and also as a desiccant due to its hygroscopic properties.
Interesting facts
Sodium is a highly reactive metal that ignites in water and does not react with oil or kerosene. So to prevent the reaction with water, it is stored in oil or kerosene.
Sodium is soft enough to be cut with a knife, and it is light enough that it can float on water.

Sodium burns with a bright yellow flame when it comes in contact with the air.
Sodium is the ninth most abundant element in the human body. The element sodium makes up roughly 0.10% to 0.15% of your body mass.
Sodium has only one stable isotope, 23Na.
Related
More elements
References
1. Natrium (Sodium) – Elementymology & Elements Multidict
2. Sodium – Los Alamos National Laboratory
3. Chemical abundances determined from meteor spectra
External links
- https://www.rsc.org/periodic-table/element/11/sodium
- https://www.britannica.com/science/sodium
- https://en.wikipedia.org/wiki/Sodium
- https://pubchem.ncbi.nlm.nih.gov/element/Sodium
- https://chemistrytalk.org/sodium-element/
- https://www.ducksters.com/science/chemistry/sodium.php
- https://www.chemicool.com/elements/sodium.html
- https://www.radiochemistry.org/periodictable/elements/11.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
