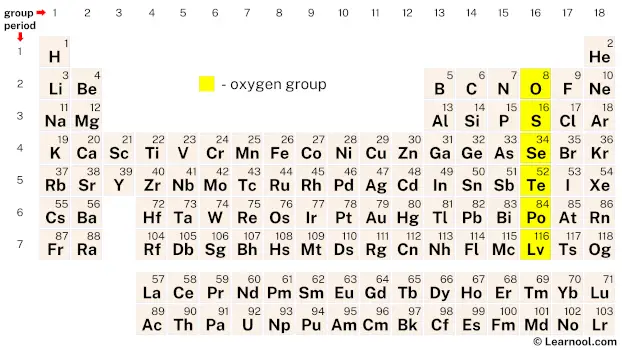
The oxygen group, also referred to as the chalcogens or oxygen family, comprises six elements on the periodic table: oxygen (O), sulfur (S), selenium (Se), tellurium (Te), polonium (Po), and livermorium (Lv). These elements exhibit varying properties with oxygen as the lightest element in the group, categorized as a reactive nonmetal along with sulfur and selenium. Tellurium is a metalloid, having characteristics of both metals and nonmetals, while polonium is classified as a post-transition metal. All elements in the oxygen group have a total of six valence electrons.
The term “chalcogen,” used to refer to the oxygen group is derived from the Greek words “chalcos,” meaning “ore,” and “-gen,” meaning “formation.” This name reflects the tendency of these elements to combine with metals to form ore-like compounds.
The oxygen group elements find diverse applications in various fields. For example, oxygen itself is essential for respiration and combustion processes. Sulfur is widely used in the production of sulfuric acid, fertilizers, and rubber. Selenium has applications in electronics, photovoltaic cells, and glass manufacturing. These elements exhibit varying levels of toxicity, with compounds like sulfur dioxide and selenium compounds being known for their harmful effects.
The elements in the oxygen group have several allotropes. For instance, oxygen exists as O2, O3 (ozone), and other forms, each with distinct properties and applications. Sulfur also has multiple allotropes, including rhombic sulfur and monoclinic sulfur. These allotropes contribute to the versatility and unique properties of the elements in the oxygen group.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – oxygen group |
On the periodic table, the oxygen group, also known as group 16, consists of six chemical elements – oxygen, sulfur, selenium, tellurium, polonium, and livermorium.
Related
More topics
- Boron group
- Carbon group
- Nitrogen group
- Oxygen group
- Halogen
- Noble gas
External links
- Oxygen group element | Chemical Properties, Uses & Reactions – Britannica
- Group 16: The Oxygen Family (The Chalcogens) – Chemistry LibreTexts
- Oxygen Group – The Periodic Table Classification Of Elements Into Groups By Electronic Structure – Jack Westin
- Chalcogen – Wikipedia
- The Oxygen Group of Elements – HyperPhysics Concepts
- Chalcogens on the Periodic Table – Oxygen Group or Group 16 – Science Notes and Projects
- The Oxygen Group | The Aqueous Chemistry of the Elements – Oxford Academic
- Group 16 The Chalcogens (Oxygen Family) – Kent’s Chemistry
- Oxygen Family – Concept – Chemistry Video – Brightstorm
- Why are oxygen and other group 16 elements called chalcogens? – Quora
- What group does oxygen belong to on the periodic table? – CK-12 Foundation
- What do the elements in the oxygen family (group 16) have in common – Brainly
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.





