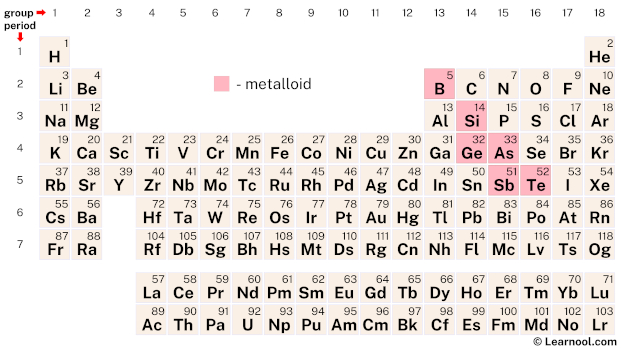
Metalloids are elements that exhibit both metallic and non-metallic properties. There are six recognized metalloids in the periodic table: boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te). These elements have properties that are intermediate between those of metals and nonmetals.
Metals are typically good conductors of electricity and heat, while nonmetals are typically poor conductors. Metalloids can behave like metals or nonmetals depending on the conditions. For example, silicon is a semiconductor, which means that it conducts electricity better than a nonmetal like sulfur, but not as well as a metal like copper.
The concept of metalloids has evolved over time, and the classification of certain elements as metalloids is still a matter of debate. In addition to the six recognized metalloids, some elements such as polonium (Po), astatine (At), and selenium (Se) are sometimes considered metalloids due to their properties.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – metalloid |
The elements – boron, silicon, germanium, arsenic, antimony, and tellurium – are the six known metalloids on the periodic table.
History

The concept of metalloids has a long and complex history. The term “metalloid” was first suggested in 1808 by Paul Erman and Paul Ludwig Simon to describe the newly discovered elements sodium and potassium, which were lighter than water and not considered proper metals by many chemists at the time. However, their proposal was largely ignored by the chemical community.
It wasn’t until 1811 that Jöns Jacob Berzelius, a Swedish chemist, used the term “metalloid” to refer to elements that exhibited properties between those of metals and nonmetals. Berzelius was referring to nonmetallic elements’ ability to form oxyanions, but the term eventually came to encompass any element with properties intermediate between metals and nonmetals.
Throughout the 19th and 20th centuries, there was much debate among chemists over which elements should be classified as metalloids. Initially, only six elements were recognized as metalloids: B, Si, Ge, As, Sb, and Te. However, the classification of other elements such as Po and At as metalloids is still a matter of debate. Some authors may consider them as metalloids due to their properties. Despite ongoing debates about the classification of certain elements, the concept of metalloids continues to be an important one in modern chemistry.
Occurrence
Most metalloids are rare in occurrence in the Earth’s crust, typically ranging from 0.01 to 0.1 parts per million in abundance. However, some metalloids, including silicon, are more abundant with an occurrence of approximately 27.7% in the Earth’s crust. Other metalloids such as germanium and arsenic have higher occurrences, with abundances of 1.6 and 1.5 parts per million, respectively.
Metalloids are found in various minerals and ores, with boron being present in borax and kernite minerals, silicon found in sand, quartz, and various types of rock, and germanium obtained as a byproduct of zinc and copper processing. Arsenic is typically found in copper, gold, and lead ores, while antimony is commonly found in stibnite ore. Tellurium is usually obtained as a byproduct of copper and lead refining.
Production
The production of metalloids varies depending on the element. For example, boron and silicon can be produced by reducing their oxides with carbon or other reducing agents. Germanium is commonly refined from zinc and copper ores, and antimony is often produced through roasting stibnite ore to form antimony oxide, which is then reduced to produce metallic antimony. Tellurium is typically obtained through various extraction methods, such as electrolysis and precipitation, as a byproduct of copper refining.
Properties
Physical properties
All metalloids are solids at room temperature.
They have intermediate conductivity, which means that they can conduct electricity to some extent but not as well as metals.
Metalloids have variable hardness, ranging from brittle to semi-hard.
Chemical properties
Metalloids have varying levels of reactivity, depending on the specific element and the conditions it is exposed to.
They have the ability to form alloys with metals, which enables the creation of useful materials.
Metalloids often have amphoteric properties, meaning that they can act as both acids and bases.
Metalloids have an intermediate number of valence electrons, which can contribute to their varying levels of reactivity and conductivity. For example, boron has 3 valence electrons, while silicon and germanium have 4, arsenic and antimony have 5, and tellurium has 6.
Optical properties
Metalloids exhibit variable optical properties, with some being transparent and others being opaque.
Due to their unique optical properties, metalloids can be used as semiconductors in electronic devices.
Applications
Semiconductors
Silicon, germanium, and other metalloids are used extensively in the electronics industry as semiconductors. These materials have unique electrical properties that allow them to be used in a range of devices, from transistors and diodes to solar cells and microchips.
Glass and ceramics
Metalloids such as boron and silicon are used in the production of glass and ceramics. Borosilicate glass, for example, is a type of glass that contains both boron and silicon, and is known for its strength, durability, and resistance to thermal shock. Silicon carbide is another ceramic material that is used in a variety of applications, from cutting tools to bulletproof vests.
Flame retardants
Antimony trioxide is a compound composed of antimony and oxygen that is used as a flame retardant in a range of materials, including plastics, textiles, and furniture.
Medicine
Arsenic, while highly toxic, has been used for medicinal purposes for centuries. It has been used to treat conditions such as syphilis and leukemia, and is still used today in some forms of chemotherapy.
Agriculture
Boron is an important nutrient for plant growth, and is used in fertilizers to improve crop yields. It is also used as a preservative in wood products, helping to prevent decay and insect damage.
Interesting facts
Metalloids are unique elements that have properties of both metals and nonmetals, making them very useful in many industries.
Boron has the highest melting point of all the metalloids, at 2076 ℃.
Some metalloids, such as silicon, are essential for life and are found in many biological systems, such as bones, teeth, and connective tissues.
Silicon, the most abundant metalloid on Earth, is used extensively in the production of computer chips and solar panels.
Germanium is used in making transistors and other electronic devices.
Metalloids have played a significant role in human history, with the ancient Chinese using arsenic as a poison and the ancient Egyptians using antimony to make cosmetic powders.
Arsenic is used in rat poisons, while its oxide is used in the manufacturing of semiconductors.
Antimony was used by ancient Egyptians to make kohl eyeliner, and its compounds are still used in the production of flame retardants and lead-acid batteries.
Tellurium is used in the production of CDs, DVDs, and Blu-ray discs.
Metalloids can be found in nature in various forms, such as in minerals, rocks, and even living organisms.
Polonium and astatine, although not universally agreed upon, are sometimes considered metalloids due to their intermediate properties between metals and nonmetals.
Metalloids can form alloys with metals, such as the well-known alloy bronze, which is made of copper and tin, and may contain small amounts of other elements like aluminum, manganese, or zinc.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Metalloid – Wikipedia
- Metalloid | Definition, Elements, & Facts – Britannica
- 6.7: Metalloids – Chemistry LibreTexts
- Metalloids: Properties and Uses – Xometry
- The Marvelous Metalloids of the Periodic Table – ChemTalk
- Metalloids – CK-12
- Metals, Metalloids, and Nonmetals – Angelo State University
- What’s the Difference Between Metals, Nonmetals, and Metalloids? – Mead Metals
- Periodic Table of the Elements – Metalloids – Gordon England
- Metalloids – Winston-Salem/Forsyth County Schools
- Metalloids – Chemistry for Kids: Elements – Ducksters
- Structure and General Properties of the Metalloids – UH Pressbooks
- Metalloid Elements | Definition, Properties & Examples – Study.com
- Metalloid – an overview – ScienceDirect
- What are Metalloids and Its Properties, Elements, and Uses – LEADRP
- Metalloid – New World Encyclopedia
- Metalloids — Overview & Properties – Expii
- Which Elements Are Metalloids? – ACS Publications
- Metalloids – Chemical Elements.com
- Elements: Metalloids – Springer
- Metalloids/Non-Metals on the Periodic Table – Laurence Lavelle
- 18.3 Structure and General Properties of the Metalloids – OpenStax
- Definition of metalloid – Chemistry Dictionary – Chemicool
- Metalloids: The Semi-Metals – ThoughtCo
- Metalloids – Chemistry Learner
- Metalloids – Chemistry – Socratic
- Elements: Metalloids – Springer
- The Periodic Table: Metals, Nonmetals, and Metalloids – Dummies
- Metalloid Definition & Meaning – Dictionary.com
- Lesson Explainer: Metals, Nonmetals, and Metalloids – Nagwa Limited
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
