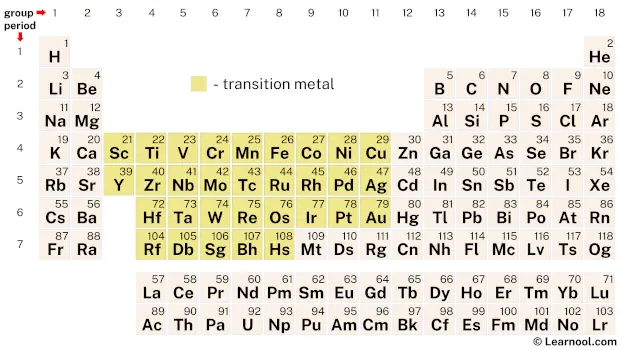
Transition metals, also known as transition elements, are a group of metallic elements that occupy the central block, or “d-block“, of the periodic table. They are characterized by their unique electronic configurations, which involve partially filled d orbitals. The transition metals include some of the most common and useful elements, such as iron, copper, nickel, and gold.
The classification of elements as transition metals is not always straightforward. While some sources consider lutetium and lawrencium to be transition metals, others exclude them from the group. Similarly, there is some debate over whether zinc, cadmium, and mercury should be included as transition metals. On the other hand, meitnerium, darmstadtium, roentgenium, and copernicium are expected to exhibit properties of transition metals. In total, there are 31 elements that are generally recognized as transition metals, although there may be some variation in the exact list depending on the source.
Transition metals are commonly found in ores and minerals, which are naturally occurring materials that contain a high concentration of these metals. The most common types of transition metal ores are oxides, sulfides, and carbonates, which are compounds that contain oxygen, sulfur, or carbon in addition to the metal element itself. For example, iron can be extracted from iron oxide ore (FeO), copper from copper sulfide ore (Cu2S), and zinc from zinc carbonate ore (ZnCO3). These ores can be mined from various sources around the world and once extracted, the transition metals can be purified and refined using a variety of chemical and physical methods.
The transition metals have a wide range of physical and chemical properties, which make them important in a variety of contexts. They have high melting and boiling points, high densities, and are generally hard and strong. They exhibit a range of oxidation states and often form colorful compounds due to their electronic configurations. They are also excellent conductors of heat and electricity, and are frequently used in electrical wiring and other electronic components.
Transition metals are widely used in many industries due to their unique properties. They are used in metallurgy to produce alloys with enhanced mechanical and chemical properties, such as increased strength, corrosion resistance, and ductility. Stainless steel, for example, is an alloy of iron, chromium, and nickel that is commonly used in the construction of buildings and infrastructure.
Transition metals, while essential and valuable, can also have negative impacts on the environment and human health. For example, some transition metals such as cobalt, chromium, and manganese can be toxic and cause health problems when exposed to high levels. The mining and processing of transition metals can lead to environmental degradation and pollution. This highlights the importance of ensuring their safe and responsible use, as well as developing effective strategies to mitigate their negative impacts on the environment and human health.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – transition metal |
The transition metals are a group of 31 chemical elements located in the central portion of the periodic table. These elements span from group 3 to group 11, and include metals such as titanium (Ti) and silver (Ag) as well as lesser-known elements like hafnium (Hf) and rhenium (Re).
History

Transition metals have a rich and fascinating history dating back to ancient times. Many of these elements were known and used by ancient civilizations, such as copper and gold by the Egyptians and silver by the Romans. However, it was not until the 18th and 19th centuries that the concept of transition metals as a distinct group of elements emerged.
During the 18th and 19th centuries, chemists began to recognize that certain metallic elements exhibited unique chemical behaviors that set them apart from the main-group of metals. For example, platinum was found to form complex compounds with ammonia, while iron could form multiple oxidation states. This led to the development of the concept of “transitional metals”, a term coined by the English chemist Charles Bury in 1921. The recognition of transition metals as a distinct group of elements was a major step forward in the understanding of the periodic table and the behavior of chemical elements.
As more research was conducted on transition metals, scientists discovered new and useful applications for these elements. For example, in the early 1900s, nickel was found to be an excellent catalyst for the hydrogenation of vegetable oils, which led to the widespread use of nickel catalysts in the food industry. In the mid-20th century, the use of transition metal catalysts in organic synthesis became a major area of research, leading to the development of new drugs, polymers, and materials.
Throughout the 20th century, scientists continued to study and discover new transition metals, as well as develop new applications for these elements. This ongoing research has made the study of transition metals a cornerstone in chemistry, with applications ranging from catalysis, electronics, medicine, and environmental science.
Occurrence and production
Transition metals are widely distributed in the Earth’s crust, with some being abundant and others being relatively rare. The most abundant transition metal is iron, followed by titanium, manganese, and zirconium. However, several transition metals such as vanadium, chromium, nickel, and copper are moderately abundant in the Earth’s crust. The abundance of these elements varies depending on the geological location.
Transition metals are found in various minerals, which are formed by geological processes. For example, chromium is found in chromite, which is a mineral composed of iron(Ⅱ) oxide and chromium(Ⅲ) oxide compounds. Copper is often found in minerals such as chalcopyrite, bornite, and malachite. Similarly, nickel is commonly found in minerals such as pentlandite, millerite, and garnierite. The abundance of transition metals in minerals is usually measured in parts per million (ppm).
The production of transition metals involves various extraction processes depending on the type of mineral and the element being extracted. For example, iron is usually extracted from iron ore using a blast furnace, while copper is often extracted through heap leaching, solvent extraction, and electrowinning. Similarly, vanadium is typically extracted from its ores through a combination of alkaline salt roasting, leaching with an acid or alkali, and precipitation with ammonium sulfate or ammonium chloride. Titanium is often extracted through the Kroll process.
Transition metals are produced as major commodities by several countries. For example, China is the largest producer of iron and manganese, while Russia is a major producer of nickel and platinum-group metals. Australia is a major producer of titanium and zirconium, while South Africa is a significant producer of chromium and vanadium. The United States is a significant producer of several transition metals, including copper, nickel, and gold.
Transition metals are being extracted more efficiently and with reduced environmental impact through the development of new technologies, alongside traditional extraction processes. For example, bioleaching involves the use of microorganisms to extract metals from ores, while hydrometallurgy uses liquid-based processes to extract metals from ores. These technologies offer potential benefits in terms of reduced environmental impact and increased efficiency.
Properties
Physical properties
Transition metals have a high melting and boiling point due to their strong metallic bonds, which require a large amount of energy to break.
They are also good conductors of heat and electricity because of the presence of free electrons in their metallic bonds, which can move freely through the metal lattice.
These metals are dense and hard due to the closely packed metallic atoms.
Despite their hardness, transition metals are still malleable and ductile, meaning they can be easily hammered or stretched into various shapes.
They also have a shiny appearance, which is due to their ability to reflect light.
Transition metals have variable oxidation states, which allow them to form various types of chemical bonds.
Transition metals are typically solid at room temperature.
Chemical properties
One of the important chemical properties of transition metals is their tendency to form coordination compounds. They can form complex ions with ligands due to their ability to accept and donate electrons.
Transition metals also have a unique ability to form colored compounds. This is because of the d-d transitions that occur in the metal’s d-orbitals.
Transition metals react with both acids and nonmetals to form a variety of ionic and covalent compounds. For instance, iron reacts with hydrochloric acid to produce iron(Ⅱ) chloride and hydrogen gas, and copper reacts with sulfur to produce copper sulfide.
Transition metals are generally resistant to corrosion due to the formation of a thin layer of oxide on their surface. This oxide layer protects the metal from further oxidation and corrosion.
They also exhibit catalytic properties, which makes them useful as catalysts in various chemical reactions.
Magnetic properties
The presence of unpaired electrons in the d-orbitals of transition metals is responsible for their magnetic properties. The number and arrangement of these unpaired electrons determine the type of magnetism exhibited by the metal. For instance, iron displays paramagnetism, copper exhibits diamagnetism, while nickel and cobalt show ferromagnetism.
Optical properties
Transition metals such as chromium and titanium have unique optical properties due to their partially filled d-orbitals. They can absorb and reflect light and are often used in pigments and dyes due to their ability to produce a wide range of colors.
Mechanical properties
Transition metals have high strength and ductility, making them suitable for a variety of industrial and structural applications.
They also have the ability to form alloys with other metals, which enhances their mechanical properties even further.
Biological properties
Many transition metals are essential trace elements in living organisms and play crucial roles in enzymes and biological processes. For instance, iron is a vital component of hemoglobin, which transports oxygen in the blood, while copper is involved in the synthesis of collagen and the production of melanin.
Despite their importance, some transition elements can be toxic to living organisms and cause severe health problems. Chromium, cobalt, and nickel are examples of toxic transition elements that can accumulate in the body and disrupt various physiological processes, leading to adverse effects on health.
Applications
Industrial
Transition metals have a variety of industrial applications due to their unique properties. For example, iron is used extensively in the construction of buildings, bridges, and vehicles due to its strength and durability. Copper is an excellent conductor of electricity, making it an essential material for electrical wiring and electronic devices. Platinum is widely used as a catalyst in chemical reactions, and is also used in jewelry due to its lustrous appearance.
Medical
Some transition metals have been investigated for their potential use in cancer treatment, such as platinum-based compounds like cisplatin and carboplatin. Several transition metals also play important roles in the human body and have medical applications. For example, iron is a key component of hemoglobin, the protein that carries oxygen in red blood cells, and copper is necessary for the function of enzymes involved in the synthesis of connective tissue.
Environmental
Transition metals are used in a variety of environmental applications. For example, iron is used in the production of stainless steel, which is used in many applications due to its durability and resistance to corrosion. Manganese is used in the production of batteries, and nickel is used in the production of rechargeable batteries. Transition metals are also used as catalysts in a variety of industrial processes, including the production of fertilizers and the removal of pollutants from wastewater.
Other

Transition metals have many other applications as well. For example, titanium is used in the production of aircraft and spacecraft due to its strength and low density. Silver is used in photography due to its sensitivity to light, and gold is used in jewelry and currency due to its rarity and durability. Some transition metals are also used in pigments, such as cobalt blue and titanium white.
Interesting facts
Transition metals are responsible for many of the colors we see in minerals and gems.
Iron is the most common transition metal and makes up much of the Earth’s core.
Silver, gold, and copper were the first metals known to humans and are all transition metals.
Many transition metals are used as catalysts in industrial processes, such as the Haber process for making ammonia.
Some transition metals, such as tungsten, are used to make filaments in incandescent light bulbs.
Transition metals are often used in electronics, such as copper in electrical wiring and silver in computer components.
Titanium is used to make strong, lightweight alloys for aircraft and spacecraft.
Many transition metals, such as iron and copper, are essential nutrients for living organisms.
Chromium is used to make stainless steel, which is resistant to corrosion and staining.
Manganese is used in the production of steel and other alloys.
Nickel is used to make coins, jewelry, and other decorative objects.
Platinum and palladium are used in catalytic converters to reduce emissions from automobiles.
Vanadium is used to strengthen steel and is found in some types of batteries.
Cobalt is used to make rechargeable batteries and is a key component in the production of magnetic alloys.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Transition metal | Definition, Properties, Elements, & Facts – Britannica
- Transition metal – Wikipedia
- Transition Metals – Purdue University
- Transition Metals – CK-12
- Transition Metals – Angelo State University
- Transition Metals — Properties of the Element Group – ThoughtCo
- 23.1: General Properties of Transition Metals – Chemistry LibreTexts
- The periodic table – transition metals (video) – Khan Academy
- Transition Metals – Chemical Elements.com
- Lecture 27: Introduction to Transition Metals | Principles of Chemical Science – MIT OpenCourseWare
- Transition Metals and Coordination Chemistry – UH Pressbooks
- Discover The World of Transition Metals – American Chemistry Council
- Transition Metals – Elements & Periodic Table – Chem4Kids
- Lesson Explainer: Transition Metals Chemistry • 7th Grade – Nagwa Limited
- Physical properties of transition elements – BBC
- introducing transition metals – Chemguide
- Transition Metals – Diamond Light Source
- 20.1: Properties of Transition Metals – Journal of Visualized Experiments
- Transition Metals Definition, List and Properties – Science Notes and Projects
- Transition Metal – an overview – ScienceDirect
- Transition Metals – Chemistry Learner
- Chemistry for Kids: Elements – Transition Metals – Ducksters
- The Transition Metals – Breaking Atom
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.

